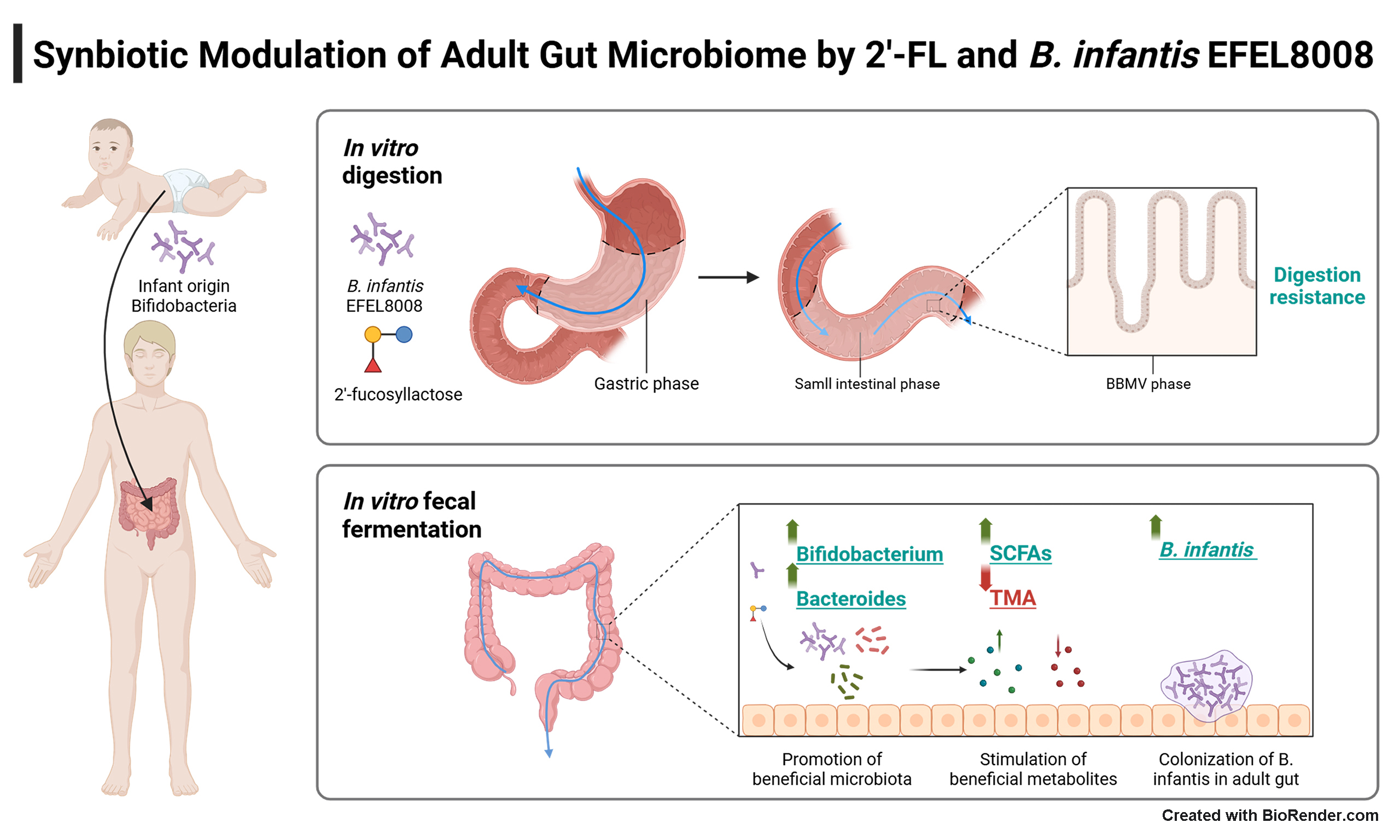
Synbiotic modulation of adult gut microbiome by 2′-fucosyllactose and Bifidobacterium longum subsp. infantis EFEL8008
Abstract
Aim: This study aimed to evaluate the combination of 2′-fucosyllactose (2′-FL) and Bifidobacterium longum subsp. infantis (B. infantis) EFEL8008 as a synbiotic pair for adult gut health, using an in vitro digestion and fecal fermentation model.
Methods: The resistance of 2′-FL to digestion was evaluated through simulated digestion encompassing oral, gastric, intestinal, and brush border membrane phases. Fecal fermentation was conducted using adult microbiota to investigate taxonomic and metabolic alterations following treatment with 2′-FL, EFEL8008, or their combination. Microbial composition was profiled using 16S rRNA gene sequencing and quantitative PCR targeting B. infantis. Short-chain fatty acids (SCFAs) and trimethylamine (TMA) levels were quantified by 1H-NMR.
Results: A total of 86.67% of 2′-FL remained intact after digestion, demonstrating its resistance to digestion throughout the upper gastrointestinal tract. The synbiotic combination significantly increased Bifidobacterium abundance and improved alpha diversity compared to single treatments. Heat tree and correlation analyses indicated selective enrichment of commensal taxa including Bifidobacterium and Lactobacillus, accompanied by a reduction in the abundance of potentially pathogenic genera such as Escherichia-Shigella. In addition, co-treatment markedly elevated the concentrations of acetate, propionate, lactate, and butyrate, and suppressed the microbial conversion of betaine to TMA, suggesting a favorable metabolic outcome.
Conclusion: These results demonstrate that the synbiotic combination of 2′-FL and EFEL8008 promotes beneficial microbial modulation, enhances metabolite production, and supports gut health, highlighting its potential as a next-generation synbiotic strategy.
Keywords
INTRODUCTION
2′-Fucosyllactose (2′-FL), one of the most predominant human milk oligosaccharides (HMOs), consists of lactose and fucose linked via an α-(1,2)-glycosidic bond[1]. It is approved as “generally recognized as safe” (GRAS) by the FDA and as a novel food by the European Food Safety Authority (EFSA). 2′-FL exhibits several bioactive properties, including prebiotic, antibacterial, antiviral, and immune-supporting effects[2,3]. It plays a crucial role in infant health by selectively supporting beneficial bacteria in the gut microbiota and has been shown to reduce diarrhea in breastfeeding infants, underscoring its importance in early life nutrition[4,5]. Recently, due to great interest in commercial applications as most promising oligosaccharides, a technology for mass production by cloning FUT2 (α-1,2-fucosyltransferase gene), a core enzyme for 2′-FL biosynthesis, has been developed using Corynebacterium glutamicum and Escherichia coli fermentation[6,7]. Therefore, the development of probiotics that can effectively use 2′-FL as a substrate represents a promising avenue for creating health-promoting synbiotics.
Bifidobacterium longum subsp. infantis (B. infantis) is a Gram-positive, heterofermentative, anaerobic bacterium that plays a pivotal role in the infant gut microbiota, particularly during the first year of life[8]. It colonizes the infant gut shortly after birth and is uniquely capable of utilizing HMOs, specifically 2′-FL, as a primary carbon source[9,10]. Unlike other bacteria that metabolize HMOs extracellularly, B. infantis internalizes them prior to degradation, reducing opportunities for cross-feeding and limiting the growth of competing microorganisms[9,11]. This distinct metabolic capability contributes to the health benefits of
A synbiotic is defined as “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host”[15]. In detail, synbiotics can beneficially affect the host by improving the survival and implantation of live microbial dietary supplements in the gastrointestinal tract[16]. Over the past decade, synbiotics have been reported to more effectively reshape the gut microbiota and confer greater health benefits compared to probiotics or prebiotics alone[17-19]. In particular, they have been associated with improvements in inflammatory responses, liver enzyme markers, insulin resistance, and lipid metabolism through modulation of functional gut microbial populations[18,19]. For instance, yogurt with Lacticaseibacillus casei or Lactobacillus helveticus combined with prebiotics such as polydextrose or lactitol has been shown to positively impact digestive health by modulating gut microbiota composition[20]. In addition, cheeses containing Lacticaseibacillus paracasei or Bifidobacterium lactis paired with fructooligosaccharides (FOS) have demonstrated benefits for digestive health[21]. Thus, developing a suitable synbiotic supplement requires careful selection to achieve either synergistic or complementary effects between probiotics and prebiotics[22]. Given this background, a synbiotic combining B. infantis and 2′-FL has strong potential to modulate the gut environment. Recent advances in microbiome research have clarified the mechanistic pathways by which probiotics and prebiotics influence host health. Probiotics modulate microbial composition and function, which in turn affects intestinal homeostasis through multiple axes, including epithelial barrier reinforcement, short-chain fatty acid (SCFA) production, and suppression of pathobionts. For example, Bifidobacterium and Lactobacillus species have been shown to upregulate tight junction proteins (e.g., occludin and ZO-1), thereby enhancing gut barrier integrity and reducing microbial translocation - key factors implicated in inflammatory bowel disease (IBD) progression[13,14]. In parallel, prebiotics such as 2′-FL promote the growth of saccharolytic bacteria that generate SCFAs, which activate host G-protein coupled receptors (GPCRs) such as FFAR2 and FFAR3. These pathways influence host lipid metabolism, glucose homeostasis, and immune modulation, providing therapeutic implications for metabolic syndrome and chronic low-grade inflammation[23,24]. Moreover, recent studies highlight that synbiotics can inhibit microbial genes involved in trimethylamine (TMA) formation, such as cutC and cntA, reducing downstream production of pro-atherogenic trimethylamine-N-oxide (TMAO)[25,26]. These mechanisms collectively underscore the potential of rational synbiotic combinations to achieve targeted modulation of the gut ecosystem and mitigate disease-related microbial pathways.
Therefore, this study aimed to evaluate the synbiotic effects of 2′-FL and B. infantis EFEL8008, using an in vitro human digestion and fermentation model. The in vitro model system offers several advantages for synbiotic research, including controlled environments that enable detailed analysis of gut-specific interactions and microbial activity. The EFEL8008 strain used in this study was originally isolated from the feces of a breastfed infant and was selected based on its robust growth on 2′-FL as a sole carbon source compared to multiple strains of Bifidobacterium, including B. infantis. To further enhance this capability, the strain was subjected to adaptive laboratory evolution (ALE), resulting in improved 2′-FL utilization[27]. To assess microbial shifts, fecal fermentation experiments were conducted under anaerobic conditions, and B. infantis was selectively quantified via RT-qPCR. In vitro fecal fermentation was subsequently conducted to analyze microbial composition, and the abundance of B. infantis was quantified by RT-qPCR. Additionally, 1H-NMR analysis was conducted to profile metabolites generated during fermentation. This study is the first to propose a synbiotic pairing of B. infantis and 2′-FL that enables stable colonization and metabolic activity in adult fecal microbiota, offering a novel strategy for next-generation synbiotic design.
METHODS
Materials and bacterial strains
The 2′-FL (purity ≥ 99%) used in this study was generously supplied by Advanced Protein Technologies Corp. (Suwon, Korea). B. infantis EFEL8008, originally isolated from infant feces, was acquired from the Korean Collection for Type Cultures (KCTC; accession number KCTC15117BP). Vitamin K1 was sourced from Wako Chemicals (Osaka, Japan). L-cysteine hydrochloride, magnesium sulfate heptahydrate (MgSO4·7H2O), bile salts, hemin, resazurin, α-amylase (from human saliva, Type IX-A), pepsin (from porcine mucosa, product code P7000), pancreatin (from porcine pancreas, product code P7545), and formic acid were obtained from Sigma-Aldrich/Merck (Darmstadt, Germany). Peptone water and yeast extract were purchased from BD Biosciences (Franklin Lakes, NJ, USA). The following inorganic salts used in the experiments - CaCl2·2H2O, K2HPO4, KH2PO4, and NaCl - were sourced from Junsei Chemical Co. (Tokyo, Japan). In addition, Tween 80 was supplied by VWR (Radnor, PA, USA). All other chemicals employed in this study were of analytical reagent grade unless stated otherwise.
Growth characteristics of B. infantis EFEL8008 with 2′-FL
To assess the growth potential of B. infantis EFEL8008 on 2′-FL as the sole carbohydrate source, the strain was cultured in 10 mL of glucose-free MRS medium supplemented with 0.05% (w/v) L-cysteine and 1%
In vitro digestibility of 2′-FL
The in vitro digestion procedure for 2′-FL was adapted with modifications from a previously established protocol[28]. For the oral digestion step, simulated salivary fluid (SSF) and α-amylase (1,500 U/mL; Type IX-A, human saliva origin, Sigma) were added to the substrate, and the reaction was maintained at 37 °C for
In vitro fecal fermentation
To investigate the impact of EFEL8008 and 2′-FL on gut microbial composition, anaerobic batch fermentation was conducted using a modified protocol adapted from a previously established method[30]. Nineteen adult volunteers who had not consumed antibiotics, probiotics, or prebiotics in the preceding three months and had no recent gastrointestinal disorders provided fresh stool samples. These samples were immediately processed upon arrival and maintained at 4 °C in sealed containers to minimize compositional shifts. All procedures involving human materials were reviewed and approved by the Institutional Review Board of Chungbuk National University (approval number: CBNU-202211-BR-0238). To prepare the inoculum, fecal matter was homogenized with PBS at a 1:10 ratio. A fermentation medium was formulated to support microbial growth, composed of peptone water (2 g/L), yeast extract (1 g/L), NaCl (0.1 g/L), phosphate salts, mineral components, bile salts (0.5 g/L), L-cysteine (0.5 g/L), hemin (50 mg/L), vitamin K1 (10 μL/L), Tween 80 (2 mL/L), and 0.05% resazurin as a redox indicator. The pH was standardized to 7.0, and the medium was pre-reduced under anaerobic conditions overnight. A total of 150 mL of the medium was inoculated with 1% (w/v) fecal slurry, and fermentation was initiated at 37 °C under constant stirring. EFEL8008 was introduced at a final concentration of 3.51 × 106 CFU/mL in the EFEL8008 and EFEL8008 +
16S rRNA amplicon-based metagenome analysis and bioinformatics analysis
Microbial composition in the fecal fermentation samples was profiled based on 16S rRNA gene amplicon sequencing using the Illumina MiSeq platform (Illumina, San Diego, CA, USA). Amplification targeted the V3-V4 regions of the 16S rRNA gene using the primer pair 341-F (5′-CCTACGGGNGGCWGCAG-3′) and 785-R (5′-GACTACHVGGGTATCTAATCC-3′)[31], in conjunction with Nextera indexing adapters. Raw sequence reads were subjected to quality trimming using Cutadapt and processed through the Deblur pipeline implemented in QIIME 2[32,33]. Taxonomic classification was carried out using a Naive Bayes classifier trained on the SILVA 132 reference database with weighted assignment. All bioinformatic analyses were performed in a dedicated Conda environment configured for QIIME 2 to ensure workflow reproducibility. Alpha diversity indices were visualized using the ggplot2 package in R, while taxonomic comparisons across groups were conducted through the MicrobiomeAnalyst online interface (https://www.microbiomeanalyst.ca/)[34]. Operational taxonomic units (OTUs) obtained from 16S rRNA gene sequencing were used for functional prediction using PICRUSt2 (v2.6.2).
Monitoring of microbial changes by real-time q-PCR
To design primers specific to B. infantis, genome-wide sequence data were examined to identify gene regions uniquely present in this subspecies. Genomic DNA was isolated from the fermentation samples using a commercial bacterial DNA extraction kit (Solgent, Daejeon, Korea) according to the supplier’s instructions, and stored at -20 °C until further use. Genes exhibiting high sequence homology to those of other bacterial taxa were eliminated based on iterative MegaBLAST searches. Among the screened targets, the gene encoding sialidase was ultimately selected for species-level discrimination. Primer pairs were constructed to yield amplicons shorter than 175 bp, consistent with recommended fragment lengths
Metabolic analysis by 1H-NMR
Metabolite profiling of fermentation samples was performed using proton nuclear magnetic resonance (1H-NMR) spectroscopy[36]. Following fermentation, each sample was centrifuged at 16,000 ×g for 10 min, and the resulting supernatant was mixed in a 1:1 ratio with deionized water containing 10% deuterium oxide and 1 mM sodium 2,2-dimethyl-4-silapentane-1-sulfonic acid (DSS), yielding a final DSS concentration of 0.5 mM. The pH of the prepared solution was adjusted to 6.00 ± 0.01 using 2 M HCl or NaOH. A volume of 700 µL was transferred into a 0.5 mm NMR tube, and spectral data were acquired using a Varian INOVA 500 MHz NMR spectrometer (Varian Inc., Palo Alto, CA, USA). Spectral identification and quantification of metabolites were conducted with the Chenomx NMR Suite (version 6.1; Chenomx Inc., Edmonton, Alberta, Canada), utilizing the Processor and Profiler modules.
Statistics
All experimental procedures were conducted in triplicate, and the resulting data are presented as mean values accompanied by standard deviations. Statistical comparisons between two groups were evaluated using independent two-sample t-tests with a two-tailed distribution. Analyses were carried out using IBM SPSS Statistics software (version 22; IBM Corp., Armonk, NY, USA). Visualization of figures was performed with GraphPad Prism software (version 8.0; GraphPad Software, San Diego, CA, USA). For core microbiome profiling, customized Python scripts (version 3.9) were utilized, incorporating functions from the pandas, seaborn, and matplotlib libraries.
RESULTS
Utilization of 2′-FL as the sole carbon source
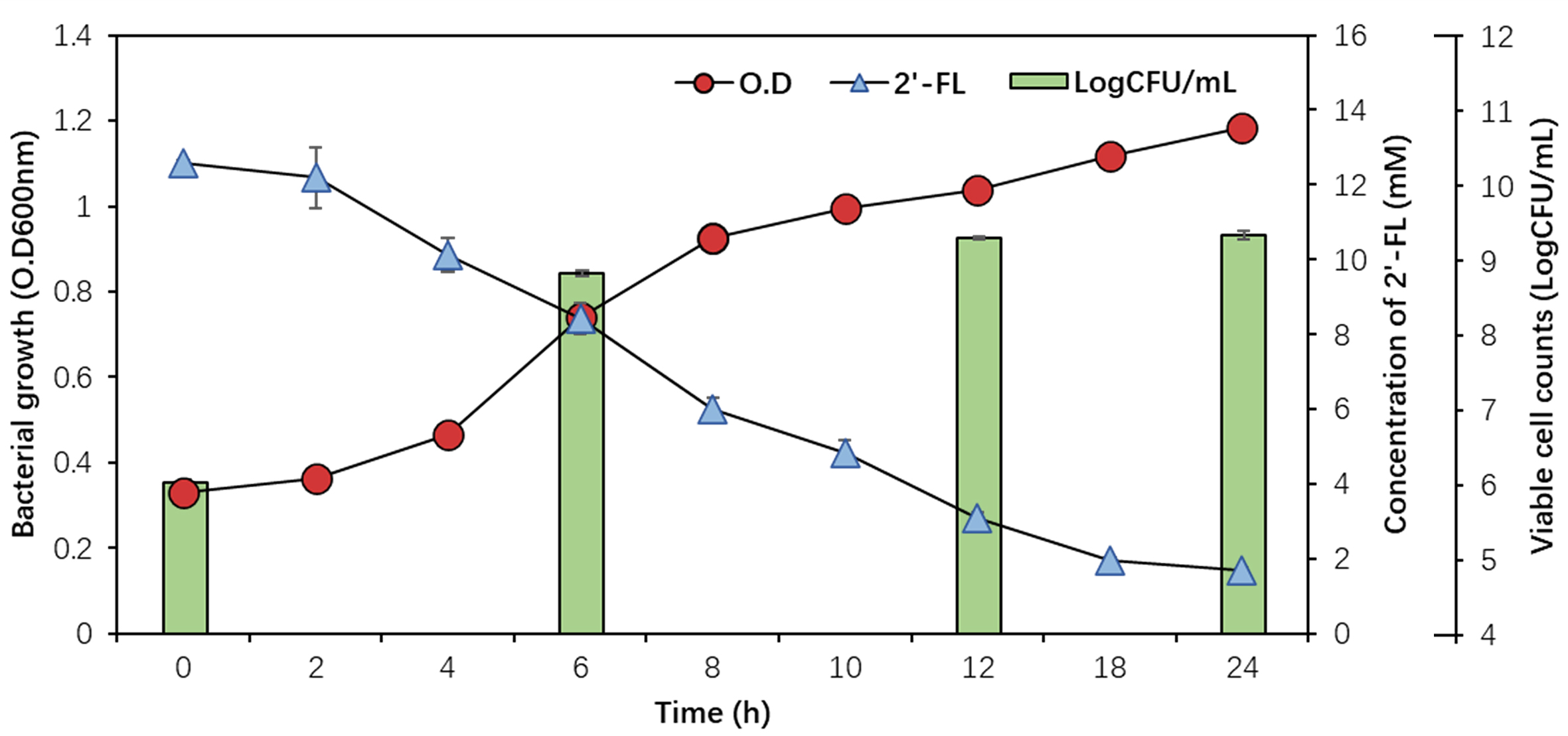
To evaluate the ability of B. infantis EFEL8008 to utilize 2′-FL, cell growth and 2′-FL consumption were measured in MRS medium supplemented with 12.59 mM of 2′-FL as the sole carbon source. As shown in Figure 1, EFEL8008 exhibited a rapid growth, reaching a population level of OD600nm 1.18 ± 0.01 and 9.34 ± 0.05 Log CFU/mL after 24 h. Simultaneously, 2′-FL concentration was decreased (12.59 ± 0.08 to 1.70 ± 0.07 mM) in the culture medium after 24 h. This result revealed that EFEL8008 effectively metabolizes 2′-FL as a carbon source, supporting robust cell proliferation.
Figure 1. Cell growth of B. infantis EFEL8008 and residual concentrations of 2′-FL. The strain was cultured at 37 °C under anaerobic conditions in MRS medium supplemented with 1% (w/v, 12.59 mM) 2′-FL as the sole carbon source. Growth was monitored over 24 h by measuring OD600nm and viable cell count (LogCFU/mL), and 2′-FL concentrations were determined by HPLC. Error bars represent the standard deviation of triplicates. B. infantis: Bifidobacterium longum subsp. infantis; 2′-FL: 2′-fucosyllactose; HPLC: high-performance liquid chromatography.
In vitro digestion assay
The in vitro digestion model was utilized to investigate the hydrolysis resistance of 2′-FL by exposing it to oral fluid, SGF, SIF, and BBMV. As a result, 2′-FL was slightly hydrolyzed during the oral (0.80% ± 0.21%), gastric (-1.20% ± 0.63%), and intestinal (2.29% ± 1.24%) phases [Table 1]. Meanwhile, 2′-FL exhibited further enzymatic susceptibility in the BBMV phase, leading to additional degradation (11.65% ± 1.74%). These results imply that 86.67% of ingested 2′-FL could reach the large intestine intact after passing through the human gastrointestinal system.
2′-FL concentration before and after in vitro digestion
| Digestion phase | 2′-FL concentration (mM) | Stepwise digestion ratioa (%) | Overall digestion ratio (%) |
| Before digestion | 44.91 ± 0.20 | 13.33 ± 2.70 | |
| After oral phase | 44.55 ± 0.12 | 0.80 ± 0.21 | |
| After gastric phase | 45.08 ± 0.25 | -1.20 ± 0.63 | |
| After small intestinal phase | 44.05 ± 0.54 | 2.29 ± 1.24 | |
| After BBMV phase | 38.92 ± 1.04 | 11.65 ± 1.74 |
Taxonomic analysis of microbial composition during in vitro fecal fermentation
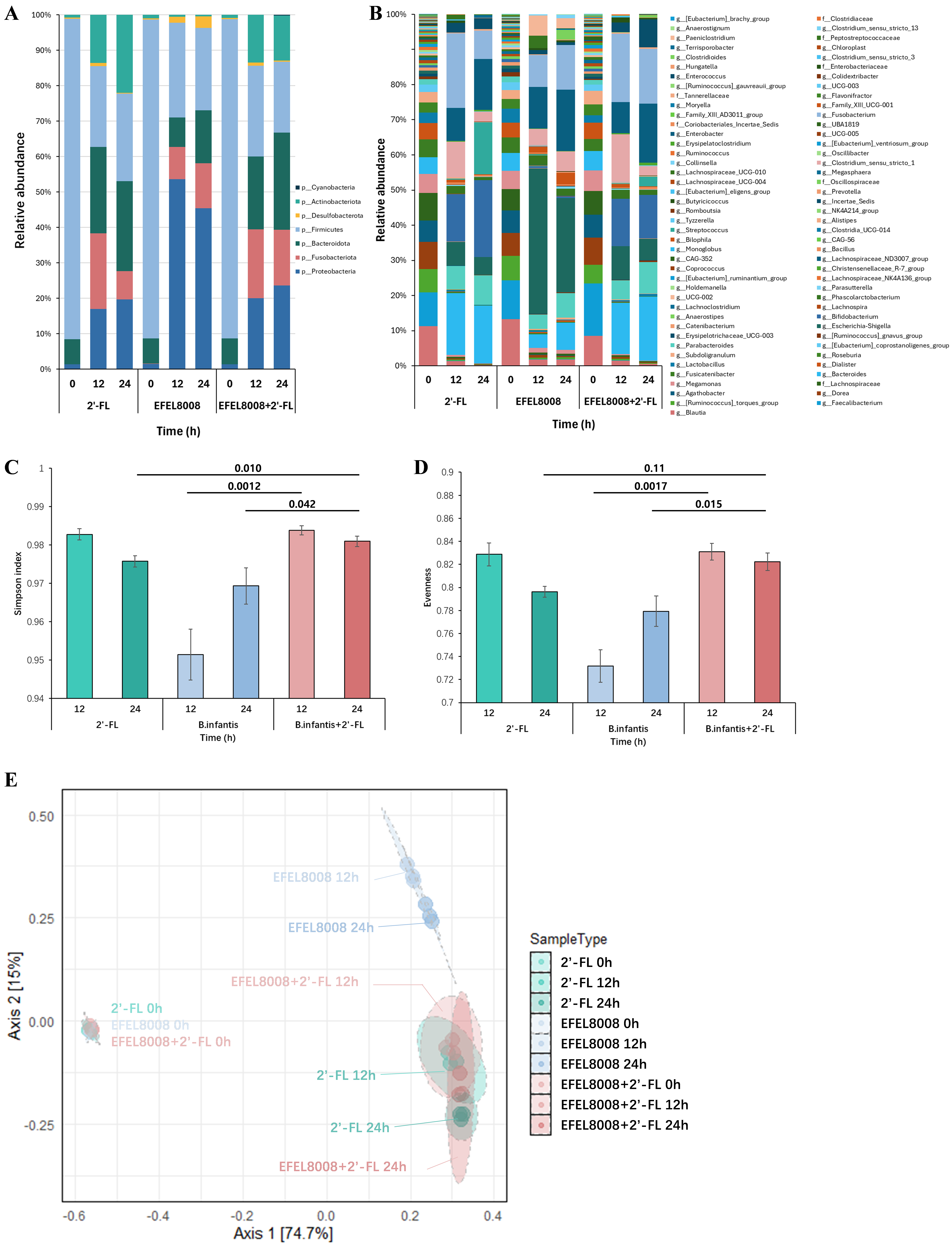
To evaluate the effects of 2′-FL and B. infantis EFEL8008 on the gut microbial composition, 16S rRNA gene-based taxonomic profiling was performed after in vitro fecal fermentation. At the phylum level, the relative abundance of Actinobacteria markedly increased in the 2′-FL (22.00% ± 1.16%) and EFEL8008 + 2′-FL (12.76% ± 3.05%) compared to the EFEL8008 (0.41% ± 0.02%) [Figure 2A]. At the genus level, Bifidobacterium was the most enriched taxon in both the 2′-FL (21.90% ± 1.15%) and EFEL8008 + 2′-FL (12.40% ± 3.06%) groups, while it remained minimal in EFEL8008 (0.39% ± 0.02%) [Figure 2B]. In terms of community structure, alpha diversity measured by the Simpson index and evenness was significantly higher in the 2′-FL and EFEL8008 + 2′-FL compared to EFEL8008 [Figure 2C and D]. Principal coordinate analysis (PCoA) based on Bray-Curtis dissimilarity further revealed distinct clustering of microbial communities, indicating that the co-administration of 2′-FL with EFEL8008 shifts both taxonomic abundance and overall diversity in a beneficial direction [Figure 2E].
Figure 2. Microbial diversity analyses and microbial abundance profiling after in vitro fecal fermentation. Bacterial taxonomic composition at the (A) phylum and (B) genus levels; Alpha diversities of (C) Simpson Index and (D) evenness, with error bars representing the standard deviation from the mean (n = 3). Significant differences between groups are indicated by P-values; (E) PCoA plot of microbial communities based on Bray-Curtis distance in two dimensions. 2′-FL, addition of 1% (w/v) 2′-fucosyllactose; EFEL8008, addition of B. infantis EFEL8008 at 3.51 × 106 CFU/mL; EFEL8008 + 2′-FL, addition of B. infantis EFEL8008 at 3.51 × 106 CFU/mL and 1% 2′-FL. PCoA: Principal coordinate analysis; 2′-FL: 2′-fucosyllactose; B. infantis: Bifidobacterium longum subsp. infantis.
Modulation of microbial interactions by synbiotic pairing
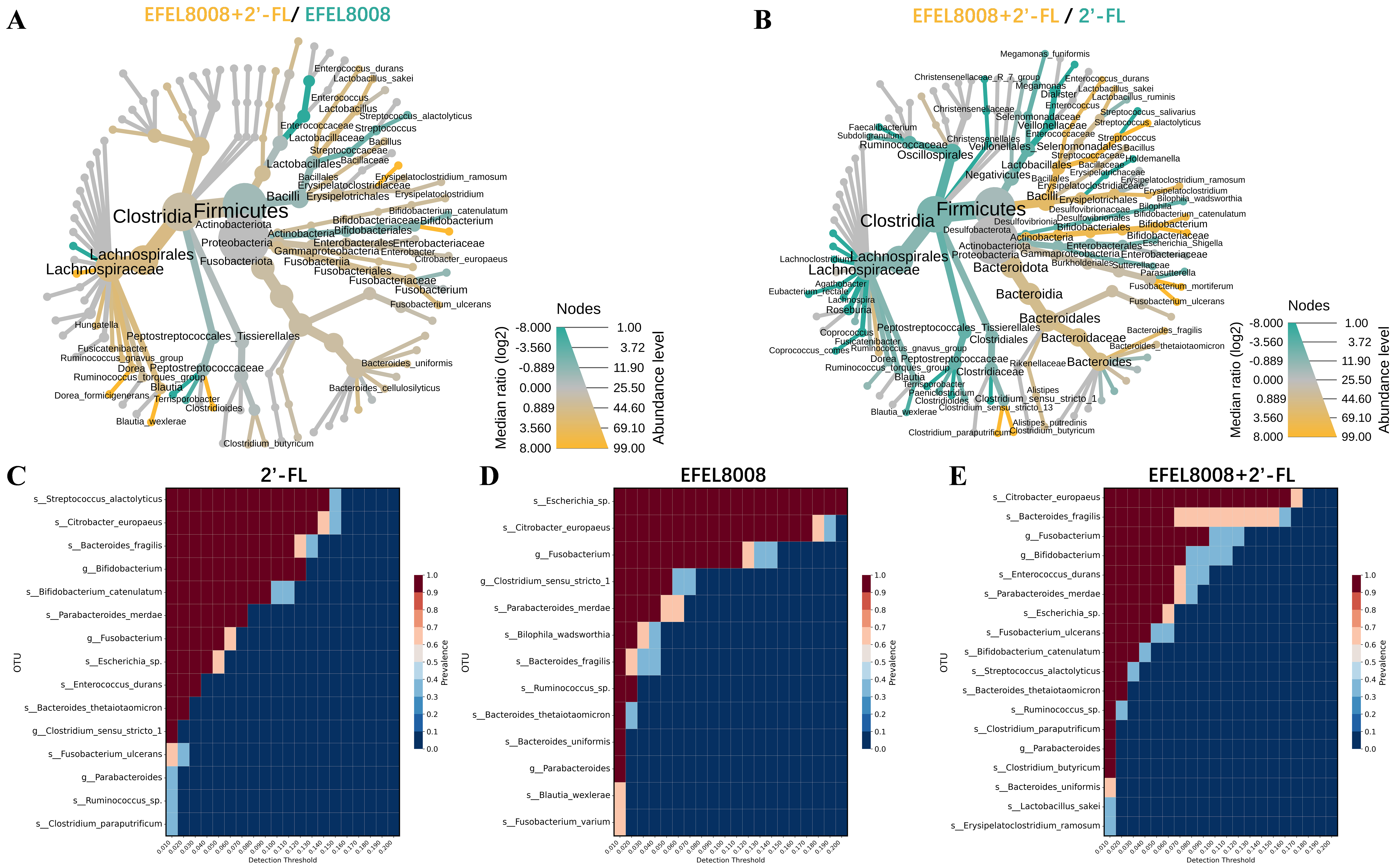
To investigate the microbial interaction patterns induced by the synbiotic combination, heat tree and core microbiome analyses were conducted. The heat tree visualization revealed that the EFEL8008 + 2′-FL exhibited a higher abundance of Bifidobacterium species compared to either EFEL8008 [Figure 3A] or 2′-FL [Figure 3B]. Notably, this group also showed a reduced abundance of potentially pathogenic taxa, such as Escherichia-Shigella, indicating a microbiota-stabilizing effect. Consistent with this, core microbiome analysis showed that Bifidobacterium was consistently enriched in the 2′-FL [Figure 3C], EFEL8008 [Figure 3D], and EFEL8008 + 2′-FL [Figure 3E] groups, with the highest abundance observed in the EFEL8008 + 2′-FL group. These observations suggest that synbiotic pairing facilitates selective engraftment of health-promoting microbes while limiting harmful species.
Figure 3. Comparison of microbial taxonomic compositions after in vitro fecal fermentation. Heat tree analysis compared microbial communities using median abundance and statistical differences based on the non-parametric Wilcoxon Rank Sum test. 2′-FL with B. infantis EFEL8008 was compared with either (A) B. infantis EFEL8008 or (B) 2′-FL at the species level after in vitro fermentation; (C-E) Core microbiome analysis showed species prevalence across detection thresholds for (C) 2′-FL, (D) B. infantis EFEL8008, and (E) 2′-FL with B. infantis EFEL8008 after in vitro fermentation. The color gradients indicated relative abundance and prevalence of the microbial species. 2′-FL, addition of 1% (w/v) 2′-fucosyllactose; EFEL8008, addition of B. infantis EFEL8008 at 3.51 × 106 CFU/mL; EFEL8008 + 2′-FL, addition of B. infantis EFEL8008 at 3.51 × 106 CFU/mL and 1% 2′-FL. 2′-FL: 2′-Fucosyllactose; B. infantis: Bifidobacterium longum subsp. infantis.
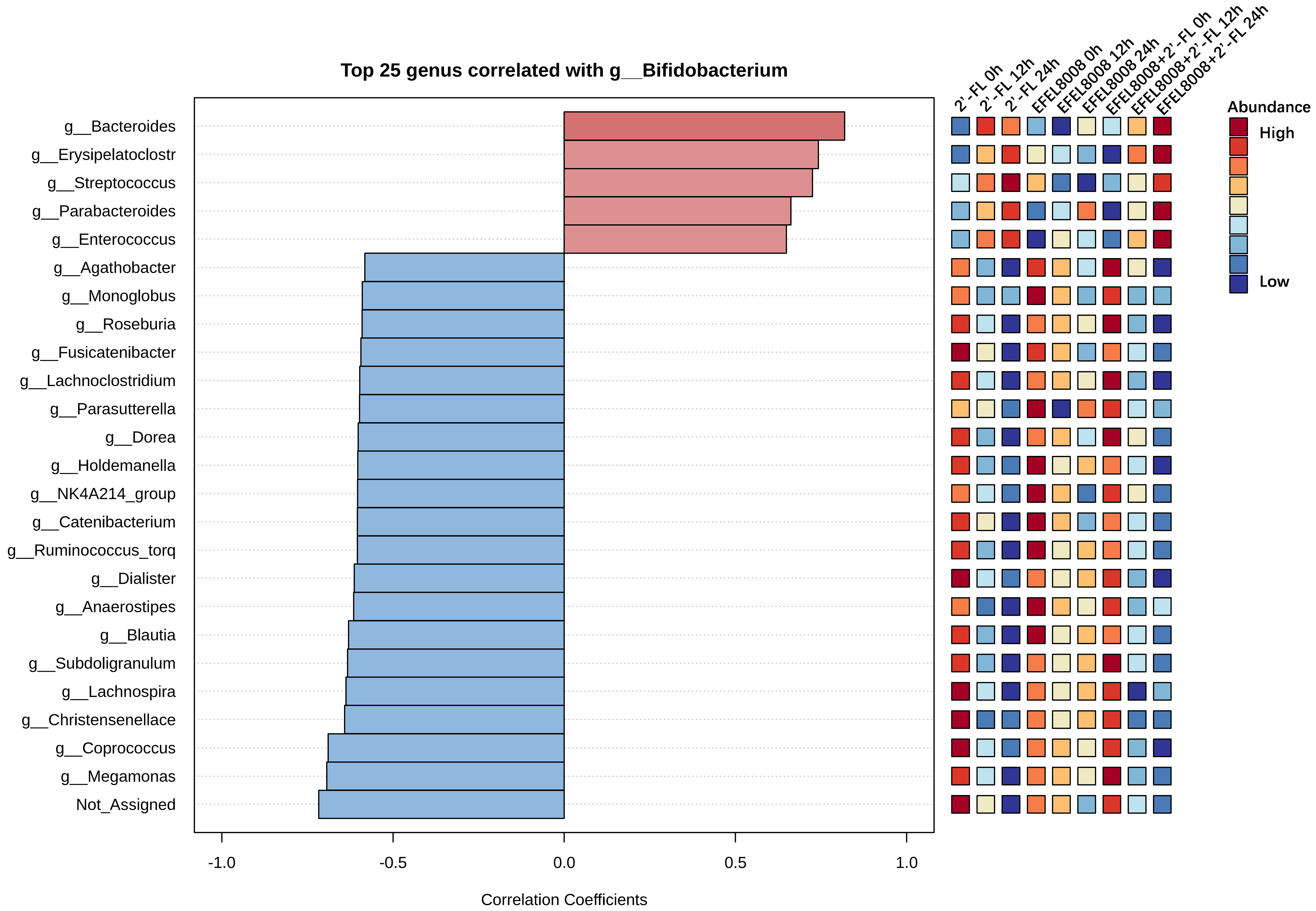
To further explore genus-level interactions, Pearson’s correlation analysis was performed using a pattern search approach centered on Bifidobacterium. As shown in Figure 4, Bifidobacterium abundance positively correlated with that of other beneficial genera such as Lactobacillus and Bacteroides in both the 2′-FL and EFEL8008 + 2′-FL. In contrast, strong negative correlations were observed with genera known to include pathogenic species, including Escherichia and Clostridium. These findings indicate that the combination of EFEL8008 and 2′-FL may contribute to a balanced microbial network by supporting commensal populations and reducing the abundance of opportunistic pathogens, with 2′-FL playing a predominant role in this effect.
Figure 4. Pearson’s correlations using pattern search that identified the top 25 genera correlated with Bifidobacterium. Blue bars indicated negative correlations, while red bars indicated positive correlations, with deeper colors representing stronger correlations. The mini heatmap on the right displayed whether the abundance of each genus was higher (red) or lower (blue) in each group. 2′-FL, addition of 1% (w/v) 2′-fucosyllactose; EFEL8008, addition of B. infantis EFEL8008 at 3.51 × 106 CFU/mL; EFEL8008 + 2′-FL, addition of B. infantis EFEL8008 at 3.51 × 106 CFU/mL and 1% 2′-FL. 2′-FL: 2′-Fucosyllactose; B. infantis: Bifidobacterium longum subsp. infantis.
Monitoring B. infantis during the in vitro fermentation
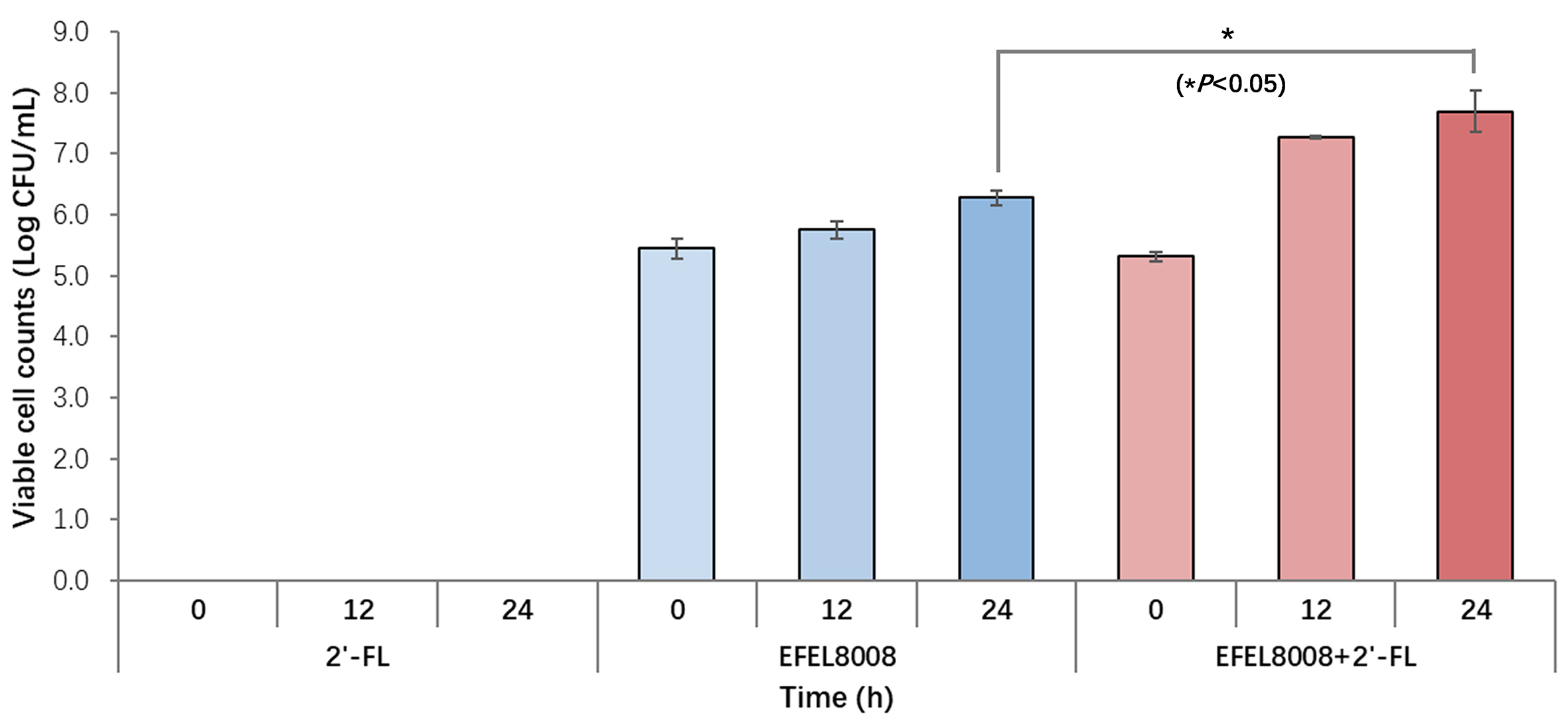
To specifically monitor B. infantis during fecal fermentation, we applied RT-qPCR methods as outlined in the Methods section. This approach enabled precise detection and targeted monitoring of B. infantis throughout the fermentation process. As shown in Figure 5, the EFEL8008 and EFEL8008 + 2′-FL, initially containing EFEL8008 at 3.51 × 106 CFU/mL, stimulated the growth of B. infantis to 6.28 Log CFU/mL and 7.69 Log CFU/mL, respectively, after 24 h of fermentation. In addition, both EFEL8008 and EFEL8008 +
Figure 5. Quantification of B. infantis during in vitro fecal fermentation. B. infantis was quantified at 0, 12, and 24 h during in vitro fecal fermentation using real-time qPCR targeting the sialidase gene. Values represent the mean ± standard deviation (n = 3). P < 0.05 compared to EFEL8008. B. infantis: Bifidobacterium longum subsp. infantis; qPCR: quantitative polymerase chain reaction.
Metabolite changes during the in vitro fecal fermentation
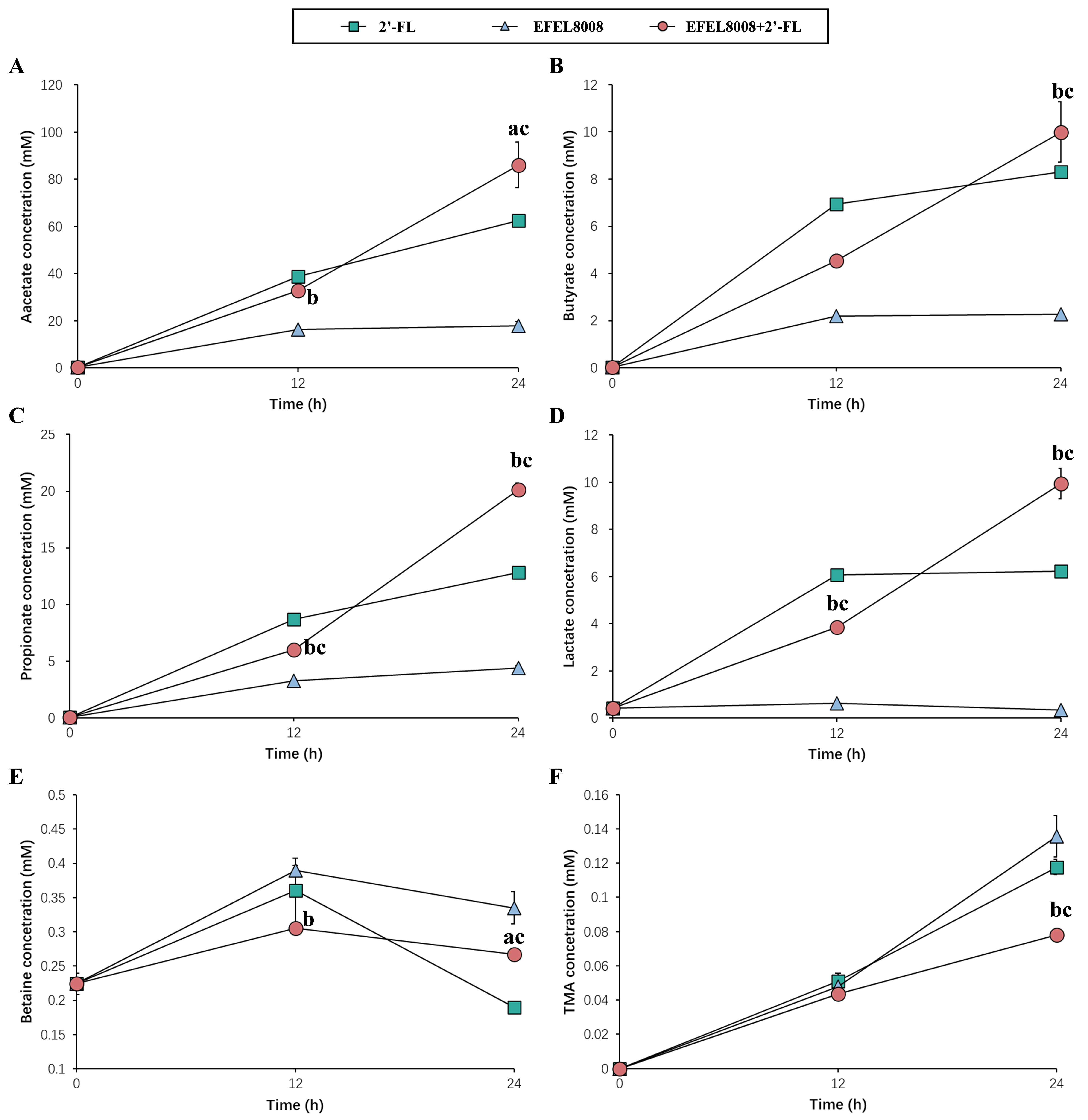
1H-NMR analysis was conducted to evaluate metabolites, including SCFAs and TMA, produced during in vitro fecal fermentation of EFEL8008 and 2′-FL. As shown in Figure 6A-D, the EFEL8008 + 2′-FL significantly increased the production of acetate (86.12 ± 9.68 mM), propionate (20.14 ± 0.58 mM), lactate (9.94 ± 0.64 mM), and butyrate (10.00 ± 1.26 mM) after 24 h of fermentation. These values were notably higher compared to those observed in the EFEL8008 (33.10 ± 1.72, 7.42 ± 0.23, 2.28 ± 0.01, and 4.20 ±
Figure 6. Changes in metabolite concentrations during in vitro fecal fermentation. (A) acetate, (B) butyrate, (C) propionate, (D) lactate, (E) betaine, and (F) TMA were analyzed. 2′-FL, addition of 1% (w/v) 2′-fucosyllactose; EFEL8008, addition of B. infantis EFEL8008 at 3.51 × 106 CFU/mL; EFEL8008 + 2′-FL, addition of B. infantis EFEL8008 at 3.51 × 106 CFU/mL and 1% 2′-FL. Error bars represent the standard deviation of triplicates. Significant differences are presented with EFEL8008 (a, P < 0.05; b, P < 0.01) or 2′-FL (c, P < 0.01). 2′-FL: 2′-Fucosyllactose; B. infantis: Bifidobacterium longum subsp. infantis.
Functional gene prediction of microbial metabolism using PICRUSt2
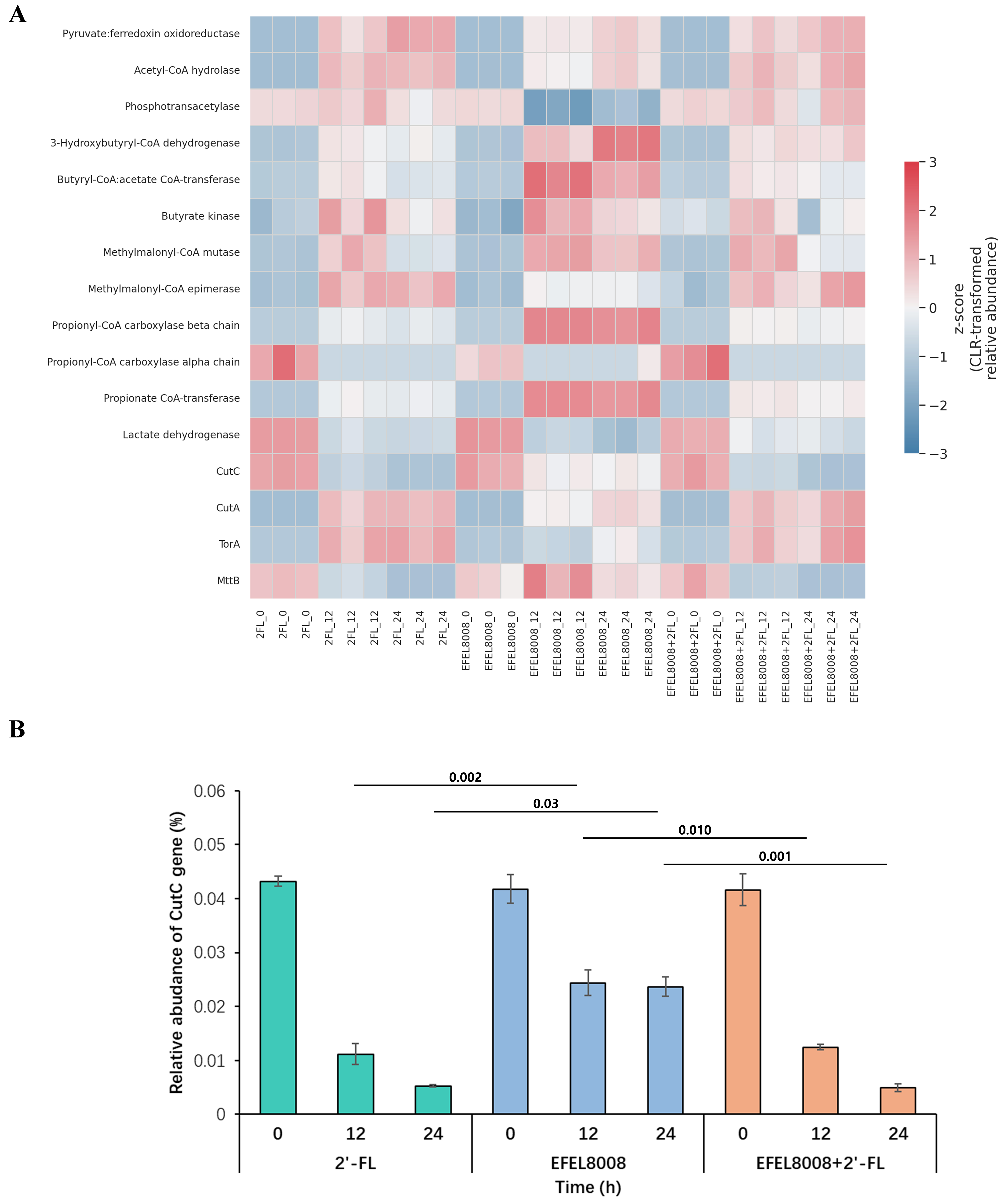
To assess potential functional changes in microbial metabolism, PICRUSt2-based gene prediction was performed using 16S rRNA sequencing data. KEGG orthologs related to SCFA metabolism were selected and visualized as a heatmap based on CLR-transformed Z-scores [Figure 7A]. Genes associated with SCFA biosynthesis, such as butyryl-CoA:acetate CoA-transferase, butyrate kinase, and propionate CoA-transferase, showed higher predicted abundance in the EFEL8008 + 2′-FL group at 24 h compared to other groups. To further explore genes linked to TMA production, the relative abundance of CutC was quantified across time points. Notably, CutC abundance was significantly lower in the EFEL8008 + 2′-FL group than in the control group at 24 h (P < 0.05; Figure 7B). While these predictions are limited to computational inference, the results suggest that the synbiotic treatment may support increased fermentative capacity and potentially suppress microbial gene abundance associated with TMA biosynthesis.
Figure 7. Functional prediction of microbial genes associated with SCFA metabolism and TMA production using PICRUSt2. (A) Heatmap showing the CLR-transformed Z-scores of KEGG orthologs related to microbial metabolic pathways during in vitro fecal fermentation. Genes involved in SCFA metabolism and TMA production are visualized across experimental groups and time points; (B) Relative abundance of the CutC gene across fermentation groups at 0, 12, and 24 h. Error bars indicate the standard deviation of three biological replicates (n = 3). Statistical significance was evaluated using t-test, and P-values are indicated above the bars. SCFA: Short-chain fatty acid; TMA: trimethylamine; CLR: centered log-ratio transformation; KEGG: Kyoto Encyclopedia of Genes and Genomes.
DISCUSSION
While the combination of probiotics and prebiotics is recognized as a promising approach to promote gut health through synbiotic effects, identifying ideal pairings that specifically enhance microbial composition and metabolic activity remains a challenge. This study demonstrates that 2′-FL plays a key role in modulating gut microbiome composition, promoting SCFA production, and reducing TMA levels under
Prebiotics are defined as non-digestible food components that selectively stimulate the growth or activity of beneficial gut bacteria, particularly in the large intestine, where they exert health benefits[16]. To provide their beneficial effects on gut health, prebiotics must withstand enzymatic hydrolysis and digestion throughout the gastrointestinal tract and reach the colon intact[37]. In this study, the gastrointestinal stability of 2′-FL was evaluated using an in vitro digestion model. As shown in Table 1, 2′-FL was slightly hydrolyzed by salivary amylase and pancreatic amylase, while a small proportion (11.60%) was further degraded by BBMV during the intestinal phase. These findings are consistent with previous reports, where approximately 5% of HMOs were digested after incubation with BBMV[29]. 2′-FL has a structure of Fuc-α-1,2-Gal-β-1,4-Glc. The α(1→2) glycosidic linkage can potentially be cleaved by BBMV enzymes[38]. However, only limited degradation occurred, likely due to low substrate specificity and restricted enzyme accessibility. These results indicate that the majority of ingested 2′-FL reaches the large intestine intact. This supports its role as an effective prebiotic.
In vitro fecal fermentation offers distinct advantages over human clinical trials. The application of in vitro fecal fermentation reduces costs, accelerates experimental outcomes, lowers labor requirements, and minimizes ethical concerns. The use of controlled experimental conditions and high reproducibility enables precise mechanistic investigations[39,40]. In vitro fecal fermentation models are considered effective tools for assessing the responses of gut microbiota to various substrates. Previous studies have demonstrated that prebiotic interventions, such as FOS, inulin, and seaweed polysaccharides, not only promote the growth of beneficial bacteria including Lactobacillus and Bifidobacterium, but also influence other aspects of the gut microbiota such as metabolite production and community composition, with patterns closely matching those observed in human clinical studies[30,41,42]. Based on these advantages, an in vitro fecal fermentation model was applied in this study to evaluate the synbiotic effects of 2′-FL and B. infantis EFEL8008. During in vitro fecal fermentation using 2′-FL as the sole carbon source, Actinobacteria and Bacteroidetes showed the greatest increases at the phylum level, while Bifidobacterium and Bacteroides were significantly enriched at the genus level [Figure 2]. In contrast, supplementation with B. infantis EFEL8008 alone, without a fermentable substrate, did not result in a significant increase in Bifidobacterium spp. These findings reflect the limitations of administering probiotics alone. Probiotic strains administered to humans are often transiently detected in feces only during and shortly after administration, failing to establish long-term colonization in the gut[43,44]. Colonization resistance, driven by competition for resources and inhibitory interactions, further hampers the ability of single probiotics to modulate the indigenous microbiota[45,46]. In contrast, supplementation with prebiotics such as 2′-FL provides targeted nutrients that may enhance the proliferation of administered probiotics within the fecal microbiota.
Prebiotic supplementation supports the colonization of beneficial strains and promotes restructuring of the gut microbiota toward a more favorable composition[47]. In this study, 2′-FL alone significantly increased the relative abundance of Bifidobacterium compared to the control during in vitro fecal fermentation. This observation aligns with previous reports demonstrating the bifidogenic effect of 2′-FL in in vitro models using fecal samples from individuals with irritable bowel syndrome (IBS)[48]. The increase in Bifidobacterium induced by 2′-FL supplementation may further support cross-feeding interactions within the gut microbial community. Bifidobacterium species, including B. infantis, possess multiple α-fucosidases capable of releasing L-fucose from 2′-FL[49,50]. The liberated L-fucose provides a carbon source for other commensals such as Bacteroides, which may explain the concurrent increase in Bacteroides abundance observed in this study [Figure 2B]. Collectively, these results highlight that synbiotic interventions combining 2′-FL and
To evaluate the synbiotic effects of EFEL8008 and 2′-FL, it was necessary to detect and monitor target microbes throughout the complex microbiome fermentation process to ensure reproducibility. For this, culture-independent methods such as RT-qPCR have been developed to enumerate individual species from complex DNA samples by quantifying the number of copies of target genes[51]. Accurate classification of
During in vitro fecal fermentation, acetate, lactate, propionate, and butyrate were identified as the major organic acids produced [Figure 6A-D]. In the human gut, lactate is typically utilized by cross-feeding bacteria such as Eubacterium hallii and Anaerostipes spp., which are often underrepresented in vitro models[55]. Despite this limitation, the measured concentrations of SCFAs still provide meaningful insights into the fermentation capacity and metabolic shifts induced by 2′-FL and EFEL8008. This observation is consistent with previous findings from continuous colon simulator models, where acetate and lactate were the predominant SCFAs produced during synbiotic fermentation[11,56]. Propionate plays an important role in weight control and glucose homeostasis by inhibiting hepatic lipogenesis and, along with acetate, stimulates free fatty acid receptor 2 (FFAR2), thereby suppressing the secretion of ghrelin, an appetite-stimulating hormone[57]. Additionally, acetate and lactate can serve as substrates for cross-feeding by other beneficial gut microbes, particularly butyrate-producing Firmicutes species such as Anaerostipes, Eubacterium, and Faecalibacterium[11]. Consistent with this mechanism, our fermentation results also demonstrated a significant increase in butyrate levels following the synbiotic treatment with 2′-FL and B. infantis EFEL8008 [Figure 6D]. The resulting increase in butyrate production is particularly important, as butyrate serves as a primary energy source for colonocytes and provides multiple health benefits, including strengthening the gut barrier, exerting anti-inflammatory effects, and inhibiting the growth of pathogenic bacteria[58]. In addition, TMA was less converted than probiotics or prebiotics after fermentation from betaine [Figure 6E and F]. Carnitine and choline can be converted by gut microbiota into TMA via betaine compound using carnitine dehydrogenase or choline dehydrogenase[25]. The reduced levels of TMA observed under the combined treatment may be attributed to the suppression of Escherichia and Clostridium, genera that are frequently associated with microbial genes responsible for TMA production, such as cutC and cntA[59,60]. This compositional shift likely contributed to the decreased microbial conversion of betaine to TMA. In support of this, functional gene prediction using PICRUSt2 revealed a significantly lower relative abundance of cutC in the EFEL8008 + 2′-FL group compared to the control at 24 h. Although this result is based on computational inference and does not confirm gene expression or enzymatic activity, it provides additional support for the potential suppression of TMA-related microbial pathways under synbiotic treatment. Betaine can be obtained from various foods, mainly regulates lipid metabolism, glucose homeostasis, and is known to have a good effect on immune regulation[61]. Numerous studies, including the present investigation, have reported that betaine is metabolized into TMA by gut-residing microbes. Once generated, TMA is absorbed through the intestinal epithelium and transported to the liver, where it is further oxidized to TMAO via hepatic flavin-containing monooxygenases. Subsequently, TMAO is systemically distributed and may accumulate in peripheral tissues or be excreted through the kidneys. This metabolite has been implicated in the development of cardiovascular diseases, including atherosclerosis and myocardial infarction[26]. Therefore, the results of this study suggest that the synbiotic strains used may have the potential to impact CVD-related factors, highlighting the importance of further research to explore these effects in more detail. These findings suggest that the synbiotic combination of EFEL8008 and 2′-FL may influence adult gut microbial metabolism under defined conditions. However, whether intentional modulation of the gut microbiota in healthy individuals is truly beneficial remains uncertain[62]. Nevertheless, the observed metabolic responsiveness indicates that infant-derived probiotics, such as EFEL8008, may functionally engage with adult microbiota without disrupting ecosystem balance. This highlights the potential of EFEL8008 and 2′-FL as a promising synbiotic strategy for microbiota-targeted applications.
This study was conducted using an in vitro fecal fermentation model, which does not fully capture the complexity of the human gastrointestinal environment. In particular, the batch fermentation system lacks continuous removal of metabolic byproducts and the activity of key cross-feeding microbes, often leading to artificial accumulation of intermediate metabolites such as lactate and a consequent reduction in pH. This study also did not include an unsupplemented control group, which may have limited the interpretation of treatment-specific effects relative to baseline microbial activity. The exclusion of an unsupplemented control group was due to the limited capacity of the batch fermentation system, which restricted the number of experimental conditions that could be implemented in parallel using the same fecal donor. Based on this constraint, treatment groups were selected according to their relevance to the study objectives, with a focus on interactions between the probiotic and prebiotic components. In future studies, an unsupplemented control group will be included to improve interpretation against baseline microbial activity. In addition, the current in vitro platform will be extended to ex vivo and in vivo models to further validate synbiotic effects under more physiologically relevant conditions. Despite these limitations, the results provide mechanistic insights into how dietary components may influence microbial metabolic outputs. Notably, the addition of 2′-FL supported the growth of B. infantis EFEL8008 and other beneficial taxa within adult fecal microbiota under fermentation conditions. While B. infantis alone showed limited proliferation, its abundance increased substantially in the presence of 2′-FL, suggesting that this specific carbohydrate source may facilitate the expansion of infant-derived strains even in adult-associated microbial environments. Furthermore, co-treatment with 2′-FL and EFEL8008 led to increased levels of acetate, propionate, and butyrate, alongside a reduction in the microbial production of TMA. These functional changes indicate that the selected combination may promote a more favorable metabolic profile in the gut environment under defined conditions. Collectively, these findings support the potential of targeted nutritional strategies to modulate the functional capacity of the adult gut microbiota.
DECLARATIONS
Authors’ contributions
Made substantial contributions to the conception and design of the study, and performed metagenomic and statistical analyses: Lee DH, Seong H, Han NS
Conducted in vitro fecal fermentation experiments and contributed to data acquisition: Lee DH, Seong H
Designed specific primers and conducted quantitative PCR: Kim SA
Drafted and revised the manuscript with intellectual input: Lee DH, Seong H, Han NS
Reviewed, edited, and approved the final version of the manuscript: Lee DH, Seong H, Kim SA, Han NS
Availability of data and materials
The data supporting this work are provided in the text. Additional raw data will be made available by the corresponding author upon reasonable request.
Financial support and sponsorship
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry(IPET) through Agricultural Microbiome R&D Program for Advancing innovative technology Program, funded by Ministry of Agriculture, Food and Rural Affairs(MAFRA) (RS-2024-00401736).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The study involving human fecal samples was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Chungbuk National University (approval number: CBNU-202211-BR-0238). Written informed consent was obtained from all participants prior to sample collection.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Zhu Y, Wan L, Li W, et al. Recent advances on 2′-fucosyllactose: physiological properties, applications, and production approaches. Crit Rev Food Sci Nutr. 2022;62:2083-92.
2. Hill DR, Chow JM, Buck RH. Multifunctional benefits of prevalent HMOs: implications for infant health. Nutrients. 2021;13:3364.
3. Zhou W, Jiang H, Wang L, Liang X, Mao X. Biotechnological production of 2′-fucosyllactose: a prevalent fucosylated human milk oligosaccharide. ACS Synth Biol. 2021;10:447-58.
4. Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012;22:1147-62.
5. Lordan C, Roche AK, Delsing D, et al. Linking human milk oligosaccharide metabolism and early life gut microbiota: bifidobacteria and beyond. Microbiol Mol Biol Rev. 2024;88:e0009423.
6. Lee WH, Pathanibul P, Quarterman J, et al. Whole cell biosynthesis of a functional oligosaccharide, 2′-fucosyllactose, using engineered Escherichia coli. Microb Cell Fact. 2012;11:48.
7. Lee YG, Jo HY, Lee DH, et al. De novo biosynthesis of 2′-fucosyllactose by bioengineered Corynebacterium glutamicum. Biotechnol J. 2024;19:e2300461.
8. Turroni F, Milani C, Ventura M, van Sinderen D. The human gut microbiota during the initial stages of life: insights from bifidobacteria. Curr Opin Biotechnol. 2022;73:81-7.
9. Gotoh A, Katoh T, Sakanaka M, et al. Sharing of human milk oligosaccharides degradants within bifidobacterial communities in faecal cultures supplemented with Bifidobacterium bifidum. Sci Rep. 2018;8:13958.
10. Locascio RG, Niñonuevo MR, Kronewitter SR, et al. A versatile and scalable strategy for glycoprofiling bifidobacterial consumption of human milk oligosaccharides. Microb Biotechnol. 2009;2:333-42.
11. Button JE, Autran CA, Reens AL, et al. Dosing a synbiotic of human milk oligosaccharides and B. infantis leads to reversible engraftment in healthy adult microbiomes without antibiotics. Cell Host Microbe. 2022;30:712-25.e7.
12. Batta VK, Rao SC, Patole SK.
13. Dargenio VN, Cristofori F, Brindicci VF, et al. Impact of Bifidobacterium longum subspecies infantis on pediatric gut health and nutrition: current evidence and future directions. Nutrients. 2024;16:3510.
14. Gavzy SJ, Kensiski A, Lee ZL, Mongodin EF, Ma B, Bromberg JS.
15. Swanson KS, Gibson GR, Hutkins R, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17:687-701.
16. Mohanty D, Misra S, Mohapatra S, Sahu PS. Prebiotics and synbiotics: recent concepts in nutrition. Food Biosci. 2018;26:152-60.
17. Ebrahimi ZS, Nasli-Esfahani E, Nadjarzade A, Mozaffari-Khosravi H. Effect of symbiotic supplementation on glycemic control, lipid profiles and microalbuminuria in patients with non-obese type 2 diabetes: a randomized, double-blind, clinical trial. J Diabetes Metab Disord. 2017;16:23.
18. Khalesi S, Johnson DW, Campbell K, et al. Effect of probiotics and synbiotics consumption on serum concentrations of liver function test enzymes: a systematic review and meta-analysis. Eur J Nutr. 2018;57:2037-53.
19. Tabrizi R, Moosazadeh M, Lankarani KB, et al. The effects of synbiotic supplementation on glucose metabolism and lipid profiles in patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Probiotics Antimicrob Proteins. 2018;10:329-42.
20. Gomez Quintero DF, Kok CR, Hutkins R. The future of synbiotics: rational formulation and design. Front Microbiol. 2022;13:919725.
21. Ranadheera C, Vidanarachchi J, Rocha R, Cruz A, Ajlouni S. Probiotic delivery through fermentation: dairy vs. non-dairy beverages. Fermentation. 2017;3:67.
22. Chan CKY, Tao J, Chan OS, Li HB, Pang H. Preventing respiratory tract infections by synbiotic interventions: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2020;11:979-88.
23. Li F, Tai L, Sun X, et al. Molecular recognition and activation mechanism of short-chain fatty acid receptors FFAR2/3. Cell Res. 2024;34:323-6.
24. Zierer J, Jackson MA, Kastenmüller G, et al. The fecal metabolome as a functional readout of the gut microbiome. Nat Genet. 2018;50:790-5.
25. Day-Walsh P, Shehata E, Saha S, et al. The use of an in-vitro batch fermentation (human colon) model for investigating mechanisms of TMA production from choline, L-carnitine and related precursors by the human gut microbiota. Eur J Nutr. 2021;60:3987-99.
26. Arias N, Arboleya S, Allison J, et al. The relationship between choline bioavailability from diet, intestinal microbiota composition, and its modulation of human diseases. Nutrients. 2020;12:2340.
27. Lee DH. Development of synbiotics with 2′-fucosyllactose and Bifidobacterium spp. and analysis of their prebiotic effects. Cheongju: Chungbuk National University; 2022. Available from: https://chungbuk.dcollection.net/srch/srchDetail/200000771040. [Last accessed on 21 Jul 2025].
28. Minekus M, Alminger M, Alvito P, et al. A standardised static in vitro digestion method suitable for food - an international consensus. Food Funct. 2014;5:1113-24.
29. Gnoth MJ, Kunz C, Kinne-Saffran E, Rudloff S. Human milk oligosaccharides are minimally digested in vitro. J Nutr. 2000;130:3014-20.
30. Seong H, Bae J, Seo JS, Kim S, Kim T, Han NS. Comparative analysis of prebiotic effects of seaweed polysaccharides laminaran, porphyran, and ulvan using in vitro human fecal fermentation. J Funct Foods. 2019;57:408-16.
31. Klindworth A, Pruesse E, Schweer T, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1.
32. Bokulich NA, Subramanian S, Faith JJ, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. 2013;10:57-9.
33. Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:852-7.
34. Chong J, Liu P, Zhou G, Xia J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat Protoc. 2020;15:799-821.
35. Baek H, Kim S, Min W, et al. A species-specific qPCR method for enumeration of Lactobacillus sanfranciscensis, Lactobacillus brevis, and Lactobacillus curvatus during cocultivation in sourdough. Food Anal Methods. 2021;14:750-60.
36. Bo B, Seong H, Kim G, Han NS. Antioxidant and prebiotic activities of Laphet, fermented tea leaves in Myanmar, during in vitro gastrointestinal digestion and colonic fermentation. J Funct Foods. 2022;95:105193.
37. Valero-Cases E, Cerdá-Bernad D, Pastor JJ, Frutos MJ. Non-dairy fermented beverages as potential carriers to ensure probiotics, prebiotics, and bioactive compounds arrival to the gut and their health benefits. Nutrients. 2020;12:1666.
38. McConnell RE, Benesh AE, Mao S, Tabb DL, Tyska MJ. Proteomic analysis of the enterocyte brush border. Am J Physiol Gastrointest Liver Physiol. 2011;300:G914-26.
39. Jorba I, Mostert D, Hermans LHL, van der Pol A, Kurniawan NA, Bouten CVC.
40. Moon JS, Li L, Bang J, Han NS. Application of in vitro gut fermentation models to food components: a review. Food Sci Biotechnol. 2016;25:1-7.
41. Lee DH, Seong H, Chang D, et al. Evaluating the prebiotic effect of oligosaccharides on gut microbiome wellness using in vitro fecal fermentation. NPJ Sci Food. 2023;7:18.
42. Pujari R, Banerjee G. Impact of prebiotics on immune response: from the bench to the clinic. Immunol Cell Biol. 2021;99:255-73.
43. Sierra S, Lara-Villoslada F, Sempere L, Olivares M, Boza J, Xaus J. Intestinal and immunological effects of daily oral administration of Lactobacillus salivarius CECT5713 to healthy adults. Anaerobe. 2010;16:195-200.
44. Lahti L, Salonen A, Kekkonen RA, et al. Associations between the human intestinal microbiota, Lactobacillus rhamnosus GG and serum lipids indicated by integrated analysis of high-throughput profiling data. PeerJ. 2013;1:e32.
45. Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016;8:52.
46. Bazanella M, Maier TV, Clavel T, et al. Randomized controlled trial on the impact of early-life intervention with bifidobacteria on the healthy infant fecal microbiota and metabolome. Am J Clin Nutr. 2017;106:1274-86.
47. Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9:1021.
48. Ryan JJ, Monteagudo-Mera A, Contractor N, Gibson GR. Impact of 2′-fucosyllactose on gut microbiota composition in adults with chronic gastrointestinal conditions: batch culture fermentation model and pilot clinical trial findings. Nutrients. 2021;13:938.
49. Sela DA, Garrido D, Lerno L, et al.
50. Bunesova V, Lacroix C, Schwab C. Fucosyllactose and L-fucose utilization of infant Bifidobacterium longum and Bifidobacterium kashiwanohense. BMC Microbiol. 2016;16:248.
51. Kim DH, Chon JW, Kim H, et al. Detection and enumeration of lactic acid bacteria, acetic acid bacteria and yeast in Kefir grain and milk using quantitative real-time PCR. J Food Saf. 2015;35:102-7.
52. Matsuo Y, Komiya S, Yasumizu Y, et al. Full-length 16S rRNA gene amplicon analysis of human gut microbiota using MinION™ nanopore sequencing confers species-level resolution. BMC Microbiol. 2021;21:35.
53. Lawley B, Centanni M, Watanabe J, et al.
54. O’Brien CE, Meier AK, Cernioglo K, et al. Early probiotic supplementation with B. infantis in breastfed infants leads to persistent colonization at 1 year. Pediatr Res. 2022;91:627-36.
55. Xie Z, He W, Gobbi A, Bertram HC, Nielsen DS. The effect of in vitro simulated colonic pH gradients on microbial activity and metabolite production using common prebiotics as substrates. BMC Microbiol. 2024;24:83.
56. Valdés-Varela L, Ruas-Madiedo P, Gueimonde M.
57. Engelstoft MS, Park WM, Sakata I, et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab. 2013;2:376-92.
58. Liu F, Li P, Chen M, et al. Fructooligosaccharide (FOS) and galactooligosaccharide (GOS) increase Bifidobacterium but reduce butyrate producing bacteria with adverse glycemic metabolism in healthy young population. Sci Rep. 2017;7:11789.
59. Rath S, Heidrich B, Pieper DH, Vital M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome. 2017;5:54.
60. Romano KA, Vivas EI, Amador-Noguez D, Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio. 2015;6:e02481.
61. Wang H, Li S, Fang S, Yang X, Feng J. Betaine improves intestinal functions by enhancing digestive enzymes, ameliorating intestinal morphology, and enriching intestinal microbiota in high-salt stressed rats. Nutrients. 2018;10:907.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].