Did we miss something important? Rethinking the effects of autophagy and mitophagy on DNA damage repair and genomic stability
Abstract
Autophagy, a cellular recycling process, plays a key role in maintaining genomic stability and regulating DNA damage repair. However, recent studies have challenged this consensus, suggesting that upregulation of autophagy may induce DNA damage and contribute to genomic instability. Notably, several investigations have demonstrated that autophagy-mediated DNA damage can occur through mechanisms involving the production of reactive oxygen species (ROS). Despite these findings, many questions remain unresolved regarding the controversial
Keywords
INTRODUCTION
Macroautophagy (hereafter referred to as autophagy) is an evolutionarily conserved process that regulates cellular homeostasis by degrading damaged intracellular components and misfolded proteins through the formation of autophagosomes and autolysosomes[1,2]. Selective autophagy is a subcategory of autophagy where specific cargoes, such as damaged organelles, are targeted for degradation. For instance, mitophagy is a selective form of autophagy that degrades mitochondria, whereas ER-phagy targets the endoplasmic reticulum for degradation in cells[3]. By breaking down ATP-enriched organelles, autophagy is also crucial for maintaining adequate ATP levels required for cell survival, particularly during nutrient stress. However, excessive autophagy can trigger cell death through mechanisms such as apoptosis and ferroptosis[4,5]. Thus, autophagy is widely recognized as a double-edged sword because its increased activity can promote either cell survival or cell death, depending on the cellular state and treatment conditions[6,7].
Dysregulation of autophagy (macroautophagy and selective autophagy) has been implicated in the pathogenesis of numerous disorders, such as cancers and Alzheimer’s disease[8,9]. Consequently, therapeutic strategies targeting autophagic modulation are being actively pursued to counteract disease progression and restore systemic health[9-11]. Therefore, understanding the regulation and effects of autophagy is essential for advancing biological knowledge and informing clinical strategies[12,13].
CURRENT CONSENSUS
Autophagy supports DNA damage repair and genomic stability
Most scientists believe that autophagy (and its upregulation) plays a key and positive role in DNA damage repair, redox homeostasis, and the maintenance of genomic stability[14-18]. Extensive evidence supports the involvement of autophagy in nucleotide excision repair (NER), base excision repair (BER),
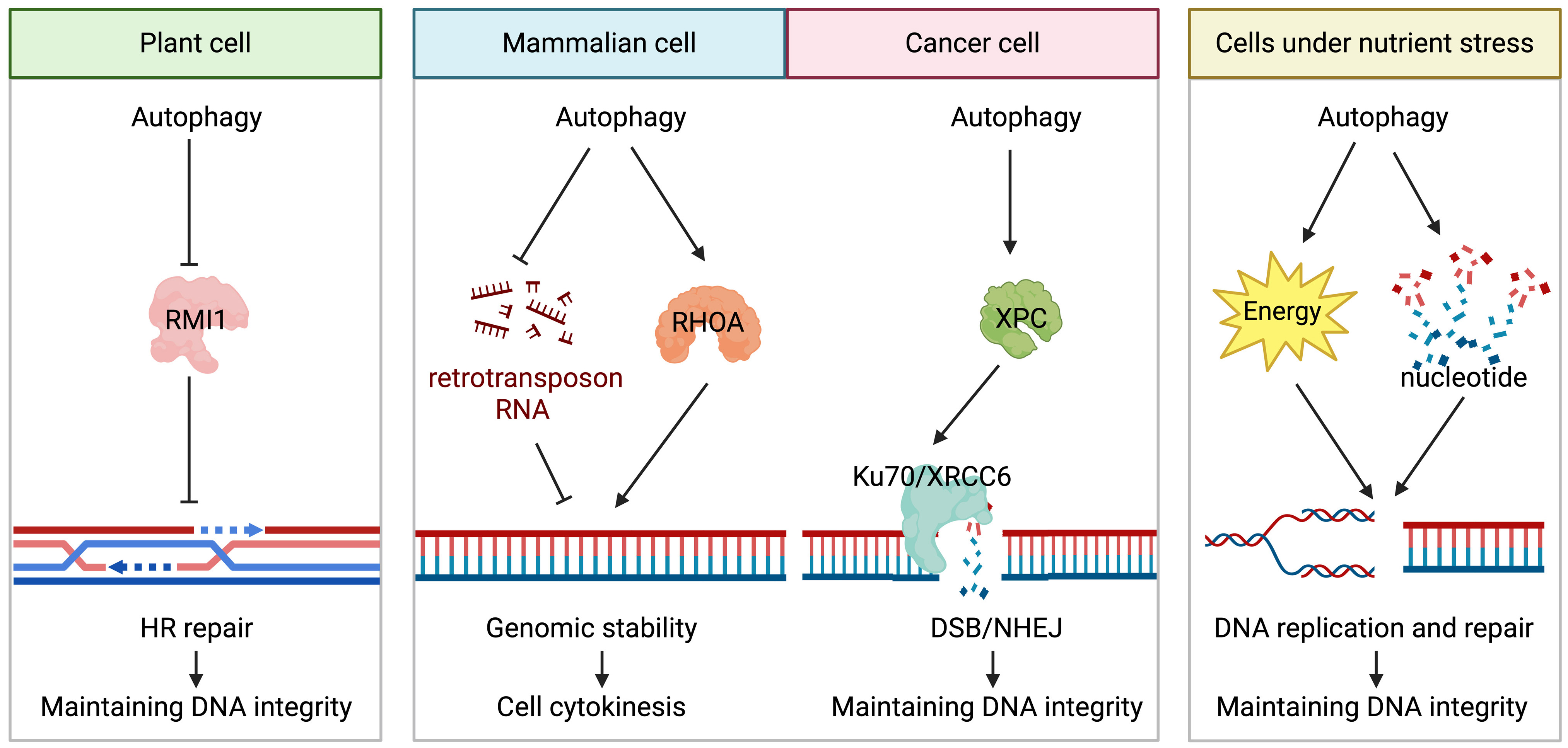
Figure 1. An illustration of the positive role of autophagy in DNA damage repair and maintenance of genomic stability. This figure highlights a portion of the process by which autophagy supports DNA damage repair and preserves genomic stability. The “→” represents “positive regulation/generation” and the “-|” represents “negative regulation/degradation”.
In plants, the autophagic degradation of RecQ-mediated genome instability protein 1 (RMI1), a component of the BTR complex responsible for double Holliday junction dissolution, promotes the HR repair of DNA inter-strand cross-links. Impaired autophagy inhibits the HR repair of DNA inter-strand cross-links and increases plant sensitivity to DNA damage[25].
CONTROVERSIES
CTSS/BIRC5-regulated autophagy-mediated DNA damage
Although autophagy is commonly activated in response to DNA damage to promote DNA damage repair, recent findings from our group and others have demonstrated that autophagy upregulation (or excessive autophagy) can induce DNA damage and genomic instability in human cancer cells and mouse embryonic fibroblasts under certain circumstances. An early study by Huang et al. showed that inhibiting cathepsin S (CTSS) by the pharmacological inhibitor 6r (an α-ketoamide-based compound) and ZFL (Z-FL-COCHO) activates autophagy and promotes intracellular ROS production in the human HONE-1 nasopharyngeal carcinoma cells[26,27]. They further demonstrated that inhibiting autophagy by inhibitors (wortmannin,
Our recent discovery provides further evidence supporting the DNA-damaging effect of autophagy. We have identified survivin (Baculoviral IAP Repeat Containing 5, BIRC5) as a novel autophagy suppressor, demonstrating that its downregulation enhances autophagy and induces genomic instability, partly via an autophagy-dependent mechanism. Despite BIRC5’s established role as an inhibitor of apoptosis[33-35], our finding shows that the small molecule BIRC5 inhibitor, YM155 (suppressing the transcription of the BIRC5 gene), induces apoptosis-independent yet autophagy-dependent DNA damage and cell death in human breast cancer cells. We demonstrated that inhibiting autophagy with pharmacological inhibitors (3-MA, chloroquine, and bafilomycin A1) or by LC3-siRNA significantly attenuates the pro-DNA-damaging effect of YM155 in various human breast cancer cell lines in vitro, including estrogen receptor-positive MCF7 breast cancer cells, tamoxifen-resistant MCF7-TamC3 and MCF7-TamR7, and the triple-negative
Radiation-induced mitophagy-mediated DNA damage
Mild and transient oxidative stress initiates mitochondrial dysfunction. While the removal of dysfunctional and damaged mitochondria via mitophagy is generally considered beneficial for cell survival[39-42], evidence also suggests that mitophagy can promote DNA damage and cell death under specific conditions[43-45]. Ren et al. reported that ionizing radiation induces ROS production and mitophagy in human PANC-1 and SW1990 pancreatic cancer cells[44]. Although the induction of ROS and mitophagy by ionizing radiation is widely observed across various cell types[46,47], their study importantly demonstrated that ionizing radiation specifically activates parkin (PRKN)/BNIP3-mediated mitophagy, thereby exacerbating DNA damage in PANC-1 and SW1990 cancer cells. The authors further showed that siRNA-mediated downregulation of PRKN and BNIP3 attenuates both the DNA-damaging and pro-cell death effects of ionizing radiation in the same cancer cell lines[44]. They also demonstrated that pharmacological activation of mitophagy with carbonyl cyanide 3-chlorophenylhydrazone (CCCP) and valproic acid (VPA) enhances ionizing
Basit et al. provided further evidence for a ROS-producing effect of mitophagy, demonstrating that mitochondrial complex I inhibition promotes a mitophagy-dependent ROS increase in melanoma cells[45]. Their research showed that inhibiting the complex I of the mitochondrial respiratory chain by the
OPEN QUESTIONS
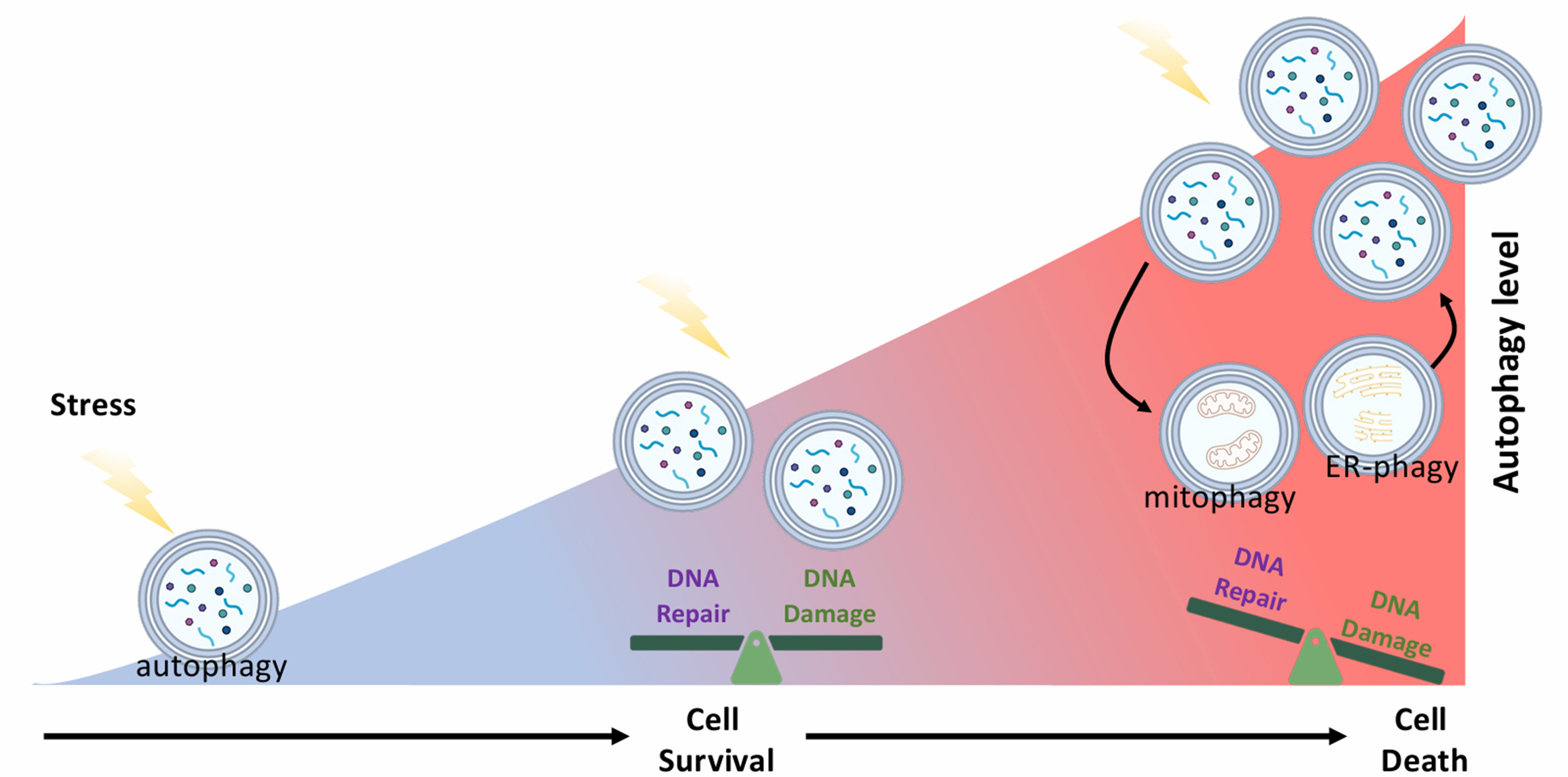
Despite significant advancements in our understanding of autophagy biology over the past two decades, several fundamental questions persist. For instance, the precise mechanisms by which autophagy shifts from a pro-survival to a pro-death role under varying circumstances remain elusive. Does autophagy upregulation promote the survival of cells under various stresses while concurrently inducing “sub-lethal” DNA damage and genomic instability (i.e., at a level of damage that is insufficient to cause cell death) in cells? Can different autophagy subtypes (e.g., the general macroautophagy, mitophagy, and ER-phagy) be simultaneously induced, subsequently engage in dynamic interactions with each other and with different intracellular molecules to generate a “unique” type of “DNA-damaging” autophagy in cells under certain conditions [Figure 2]? On the other hand, sub-lethal genotoxicity and repetitive DNA damage repair are known drivers of permanent genomic mutations. DNA damage repair mechanisms, such as NHEJ, are inherently error-prone, often leading to the introduction of mutations following repair. Notably, even low levels of oxidative stress have been shown to induce clustered DNA lesions, leading to NHEJ-mediated gene mutations in cells[48]. Could repetitive sub-lethal autophagy be one of the contributing causes of gene mutations, consequently promoting the generation of intratumoral heterogeneity? These questions are highly relevant given that cancer cells, particularly those situated in poorly vascularized regions of a tumor, frequently experience intermittent sub-lethal nutrient stress, a known activator for autophagy.
Figure 2. Differential effects of autophagy on DNA damage repair and genomic stability at different autophagy levels. Schematic diagram showing the possible simultaneous interplay between different autophagy subtypes and their spatiotemporal effects on DNA damage repair in cells. Different types of autophagy (e.g., macroautophagy, mitophagy, and ER-phagy) can be induced and interact with each other, as well as with the surrounding intracellular molecular networks, resulting in differential effects on DNA damage repair and genomic stability, at different stages in cells under nutrient stress.
FUTURE DIRECTIONS
As previously discussed[49], the impact of autophagy on genome stability and cell viability likely varies with its activity levels [Figure 2]. The dynamic interplay between different autophagy subtypes further complicates our understanding of their diverse roles in DNA damage repair. Consequently, simply measuring the end effect of autophagy (such as cell survival and cell death; the presence or absence of DNA damage) is insufficient. Future investigations require a series of carefully designed “multiple time points” and “multiple treatment-amplitude” experiments. This approach will enable a comprehensive analysis of the dynamic and spatiotemporal effects of autophagy, particularly the contributions of sub-lethal autophagy in cells. To determine whether sub-lethal autophagy and the associated DNA damage promote intratumoral heterogeneity, advanced methodologies such as single-cell sequencing or spatial transcriptomics are required.
A more comprehensive understanding of the mechanisms and implications of this “autophagy-mediated sub-lethal DNA damage” will offer crucial new insights into the development and progression of various diseases in the future. On the other hand, the development of autophagy modulators as therapeutics is a rapidly advancing research area[50-52]. A deeper insight into autophagy mechanisms will help identify potential adverse effects of autophagy-targeted interventions and clarify the molecular basis of side effects observed in various therapies.
DECLARATIONS
Authors’ contributions
Material preparation, conceptualization, visualization, writing - original draft: Cheng SM, Chang YC
Conceptualization: Lin TY, Coumar MS
Conceptualization and writing - original draft and proofreading: Leung E
Material preparation, conceptualization, funding acquisition, supervision, writing - original draft and proofreading: Cheung CHA
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the National Science and Technology Council (NSTC 113-2320-B-006-016), Taiwan.
Conflicts of interest
Cheung CHA is an Editorial Board member of Journal of Cancer Metastasis and Treatment. Cheung CHA was not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Ishida Y, Nagata K. Autophagy eliminates a specific species of misfolded procollagen and plays a protective role in cell survival against ER stress. Autophagy. 2009;5:1217-9.
2. Lee E, Koo Y, Ng A, et al. Autophagy is essential for cardiac morphogenesis during vertebrate development. Autophagy. 2014;10:572-87.
3. Bernales S, Schuck S, Walter P. ER-phagy: selective autophagy of the endoplasmic reticulum. Autophagy. 2007;3:285-7.
4. Dang S, Yu ZM, Zhang CY, et al. Autophagy promotes apoptosis of mesenchymal stem cells under inflammatory microenvironment. Stem Cell Res Ther. 2015;6:247.
5. Hou W, Xie Y, Song X, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425-8.
6. Paillas S, Causse A, Marzi L, et al. MAPK14/p38α confers irinotecan resistance to TP53-defective cells by inducing survival autophagy. Autophagy. 2012;8:1098-112.
7. Gilardini Montani MS, Santarelli R, Granato M, et al. EBV reduces autophagy, intracellular ROS and mitochondria to impair monocyte survival and differentiation. Autophagy. 2019;15:652-67.
8. Lu H, Li G, Liu L, Feng L, Wang X, Jin H. Regulation and function of mitophagy in development and cancer. Autophagy. 2013;9:1720-36.
9. Zhang Z, Yang X, Song YQ, Tu J. Autophagy in Alzheimer’s disease pathogenesis: therapeutic potential and future perspectives. Ageing Res Rev. 2021;72:101464.
10. Zhou Y, Huang Y, Liang H, et al. Resveratrol inhibits autophagy in cardiomyocytes subjected anoxia/reoxygenation injury: involved in VDAC1/PINK1/Parkin pathway. Toxicol Appl Pharmacol. 2025;502:117421.
11. Ren Z, Song Y, Xian J, et al. Identification of Fangchinoline as a novel autophagy inhibitor with an adjuvant of chemotherapy against lung cancer. Toxicol Appl Pharmacol. 2023;477:116679.
12. Wu H, Chen H, Ding X, et al. Identification of autophagy-related signatures in doxorubicin-induced cardiotoxicity. Toxicol Appl Pharmacol. 2024;491:117082.
13. Zhou G, Tang S, Yang L, et al. Effects of long-term fluoride exposure on cognitive ability and the underlying mechanisms: role of autophagy and its association with apoptosis. Toxicol Appl Pharmacol. 2019;378:114608.
14. Hewitt G, Korolchuk VI. Repair, reuse, recycle: the expanding role of autophagy in genome maintenance. Trends Cell Biol. 2017;27:340-51.
15. Bu W, Hao X, Yang T, et al. Autophagy contributes to the maintenance of genomic integrity by reducing oxidative stress. Oxid Med Cell Longev. 2020;2020:2015920.
16. Gomes LR, Menck CFM, Leandro GS. Autophagy roles in the modulation of DNA repair pathways. Int J Mol Sci. 2017;18:2351.
17. Gillespie DA, Ryan KM. Autophagy is critically required for DNA repair by homologous recombination. Mol Cell Oncol. 2016;3:e1030538.
18. Feng Y, Klionsky DJ. Autophagy regulates DNA repair through SQSTM1/p62. Autophagy. 2017;13:995-6.
19. Qiang L, Zhao B, Shah P, Sample A, Yang S, He YY. Autophagy positively regulates DNA damage recognition by nucleotide excision repair. Autophagy. 2016;12:357-68.
20. Sharma A, Alswillah T, Kapoor I, et al. USP14 is a deubiquitinase for Ku70 and critical determinant of non-homologous end joining repair in autophagy and PTEN-deficient cells. Nucleic Acids Res. 2020;48:736-47.
21. Liu EY, Xu N, O’Prey J, et al. Loss of autophagy causes a synthetic lethal deficiency in DNA repair. Proc Natl Acad Sci U S A. 2015;112:773-8.
22. Guo H, Chitiprolu M, Gagnon D, et al. Autophagy supports genomic stability by degrading retrotransposon RNA. Nat Commun. 2014;5:5276.
23. Belaid A, Cerezo M, Chargui A, et al. Autophagy plays a critical role in the degradation of active RHOA, the control of cell cytokinesis, and genomic stability. Cancer Res. 2013;73:4311-22.
24. Mathew R, Kongara S, Beaudoin B, et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21:1367-81.
25. Chen P, De Winne N, De Jaeger G, Ito M, Heese M, Schnittger A. KNO1-mediated autophagic degradation of the Bloom syndrome complex component RMI1 promotes homologous recombination. EMBO J. 2023;42:e111980.
26. Chen JC, Uang BJ, Lyu PC, et al. Design and synthesis of α-ketoamides as cathepsin S inhibitors with potential applications against tumor invasion and angiogenesis. J Med Chem. 2010;53:4545-9.
27. Huang CC, Chen KL, Cheung CHA, Chang JY. Autophagy induced by cathepsin S inhibition induces early ROS production, oxidative DNA damage, and cell death via xanthine oxidase. Free Radic Biol Med. 2013;65:1473-86.
28. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509-17.
29. Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997;94:514-9.
30. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909-50.
31. Ricci C, Pastukh V, Leonard J, et al. Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am J Physiol Cell Physiol. 2008;294:C413-22.
32. Huang CC, Lee CC, Lin HH, et al. Autophagy-regulated ROS from xanthine oxidase acts as an early effector for triggering late mitochondria-dependent apoptosis in cathepsin S-targeted tumor cells. PloS One. 2015;10:e0128045.
33. Sarvagalla S, Lin T, Kondapuram SK, Cheung CHA, Coumar MS. Survivin - caspase protein-protein interaction: experimental evidence and computational investigations to decipher the hotspot residues for drug targeting. J Mol Struct. 2021;1229:129619.
34. Shin S, Sung BJ, Cho YS, et al. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. 2001;40:1117-23.
35. Cheung CHA, Chang YC, Lin TY, Cheng SM, Leung E. Anti-apoptotic proteins in the autophagic world: an update on functions of XIAP, Survivin, and BRUCE. J Biomed Sci. 2020;27:31.
36. Cheng SM, Chang YC, Liu CY, et al. YM155 down-regulates survivin and XIAP, modulates autophagy and induces autophagy-dependent DNA damage in breast cancer cells. Br J Pharmacol. 2015;172:214-34.
37. Lin TY, Chan HH, Chen SH, et al. BIRC5/Survivin is a novel ATG12-ATG5 conjugate interactor and an autophagy-induced DNA damage suppressor in human cancer and mouse embryonic fibroblast cells. Autophagy. 2020;16:1296-313.
38. Cheng SM, Lin TY, Chang YC, Lin IW, Leung E, Cheung CHA. YM155 and BIRC5 downregulation induce genomic instability via autophagy-mediated ROS production and inhibition in DNA repair. Pharmacol Res. 2021;166:105474.
39. Kurihara Y, Kanki T, Aoki Y, et al. Mitophagy plays an essential role in reducing mitochondrial production of reactive oxygen species and mutation of mitochondrial DNA by maintaining mitochondrial quantity and quality in yeast. J Biol Chem. 2012;287:3265-72.
40. Alan P, Vandevoorde KR, Joshi B, et al. Basal Gp78-dependent mitophagy promotes mitochondrial health and limits mitochondrial ROS. Cell Mol Life Sci. 2022;79:565.
41. Frank M, Duvezin-Caubet S, Koob S, et al. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta. 2012;1823:2297-310.
42. Peng C, Li X, Ao F, et al. Mitochondrial ROS driven by NOX4 upregulation promotes hepatocellular carcinoma cell survival after incomplete radiofrequency ablation by inducing of mitophagy via Nrf2/PINK1. J Transl Med. 2023;21:218.
43. Zhang C, Nie P, Zhou C, et al. Oxidative stress-induced mitophagy is suppressed by the miR-106b-93-25 cluster in a protective manner. Cell Death Dis. 2021;12:209.
44. Ren Y, Yang P, Li C, et al. Ionizing radiation triggers mitophagy to enhance DNA damage in cancer cells. Cell Death Discov. 2023;9:267.
45. Basit F, van Oppen LM, Schöckel L, et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017;8:e2716.
46. Yamamori T, Yasui H, Yamazumi M, et al. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radic Biol Med. 2012;53:260-70.
47. Cui J, Wang TJ, Zhang YX, She LZ, Zhao YC. Molecular biological mechanisms of radiotherapy-induced skin injury occurrence and treatment. Biomed Pharmacother. 2024;180:117470.
48. Sharma
49. Cheng SM, Shieh MC, Lin TY, Cheung CHA. The “Dark Side” of autophagy on the maintenance of genome stability: does it really exist during excessive activation? J Cell Physiol. 2022;237:178-88.
50. Eshraghi M, Ahmadi M, Afshar S, et al. Enhancing autophagy in Alzheimer’s disease through drug repositioning. Pharmacol Ther. 2022;237:108171.
51. Liu Y, Zhou H, Yin T, et al. Quercetin-modified gold-palladium nanoparticles as a potential autophagy inducer for the treatment of Alzheimer’s disease. J Colloid Interface Sci. 2019;552:388-400.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].