Integrated environmental surveillance: the role of wastewater, air, and surface microbiomes in global health security
Abstract
In recent years, particularly following the COVID-19 pandemic, wastewater-based epidemiology (WBE) has emerged as an effective tool for the early detection of disease outbreaks. This manuscript presents a novel perspective on WBE by highlighting sewage as a predictive instrument, capable of providing near-real-time, community-level pathogen surveillance and anticipating and mitigating future pandemics even before the first clinical symptoms are detected. This approach enables cost-effective, non-invasive, and population-wide monitoring of infectious diseases’ emergence, evolution, and decline. By identifying pathogens in human waste (e.g., viruses and bacteria), WBE delivers real-time insights into infection trends, encompassing data from asymptomatic and pre-symptomatic populations, enabling timely interventions from public health authorities. Among the key advantages are its capacity to encompass large populations, pinpoint transmission hotspots, and facilitate resource allocation for containment efforts. The efficacy of sewage surveillance in predicting infection has already been validated during the COVID-19 pandemic, highlighting its potential as a critical component of pandemic response preparedness. However, this approach also presents challenges such as sample variability, environmental factors, and infrastructure limitations. Through a comprehensive review of the state-of-art available on this topic, including almost 300 published papers, the present manuscript emphasizes the expected impact of integrating sewage monitoring into global health surveillance frameworks and discusses its future applications in mitigating emerging infectious diseases, aiming to provide a multidimensional overview of WBE and its integration with other environmental surveillance tools.
Keywords
INTRODUCTION
The unprecedented impact of recent pandemics, such as SARS, H1N1, and COVID-19, highlights critical vulnerabilities in global health surveillance systems[1]. These events emphasize the need for innovative and scalable approaches to monitor infectious disease dynamics and to identify outbreaks before they escalate into public health emergencies[1]. Moreover, the importance of data collection, model development, empirical analysis, team science, and public trust has become evident from earlier experiences[2]. The rapid spread of SARS-CoV-2, which went undetected in multiple regions until clinical cases overwhelmed healthcare systems, serves as a reminder of how conventional surveillance methods are often limited in identifying early signals of an emerging threat[3].
Traditional disease monitoring approaches rely heavily on clinical diagnostics, which are inherently reactive and typically detect infections only after symptoms manifest[4]. This limitation creates a critical gap in early detection, particularly for asymptomatic or pre-symptomatic individuals who unknowingly contribute to community transmission[5]. Additionally, delays in sample collection, testing, reporting, and challenges in accessing healthcare facilities further exacerbate this problem, especially in resource-limited settings, such as low- and medium-income countries (LMICs)[6,7]. It is imperative to explore alternative surveillance mechanisms that provide near real-time, community-level information on disease prevalence to mitigate the risks of delayed interventions[1].
Wastewater-based epidemiology (WBE) originated in the early 2000s. Initially, it was developed to monitor illicit drug usage among communities by analyzing sewage chemical residues[8]. It has later expanded to track antibiotic-resistant bacteria and genes, providing a more comprehensive approach to assist in public health monitoring[9]. The transformative transition for WBE was observed during the COVID-19 pandemic, where it has been rapidly adopted for pathogen surveillance by detecting viral RNA, mainly SARS-CoV-2, in sewage systems and showcasing its ability to act as an early warning of outbreaks - even before clinical cases were reported - through community-level health monitoring[10]. More recently, WBE has evolved into a critical, cost-effective, and scalable public health surveillance tool by detecting various biological markers [e.g., viruses, bacteria, protozoa, and antimicrobial resistance (AMR) genes][11]. Additionally, recent technological advancements [e.g., reverse transcription quantitative polymerase chain reaction (RT-qPCR) and metagenomic sequencing] have enhanced detection capabilities[11]. Therefore, WBE constitutes a cornerstone of integrated surveillance systems for worldwide pandemic preparedness and environmental health monitoring.
This tool has emerged as a transformative tool for addressing these challenges. Sewage sample analysis utilizes molecular markers to detect pathogens shed in human waste, constituting a cost-effective, non-invasive, and population-wide surveillance method[12-14]. During the COVID-19 pandemic, WBE demonstrated its potential to detect viral RNA in sewage weeks before spikes in clinical cases, which offered health authorities a lead time to implement containment measures[13,15]. This capability extends beyond respiratory viruses, encompassing a broad spectrum of pathogens, including bacteria, viruses, and AMR genes, making WBE a versatile platform for monitoring diverse public health threats[12,16].
The utility of WBE lies in its ability to detect pathogens and its scalability and adaptability to various settings[13]. Contrary to individual testing, which is labor-intensive and logistically challenging, wastewater sampling enables community-wide health assessments using a single composite sample[17]. Additionally, its non-invasive nature makes it acceptable and practical for population surveillance, particularly in regions where healthcare infrastructure is limited or under strain. Ultimately, by detecting asymptomatic and pre-symptomatic patients and identifying trends in pathogen prevalence, WBE provides a more comprehensive understanding of disease dynamics compared to traditional surveillance methods[18].
Integrating WBE with complementary approaches, such as Aerobiology and microbial analysis of frequently touched surfaces, enhances its effectiveness[9]. Aerobiology focuses on airborne pathogen dynamics, offering valuable insights into the transmission of respiratory diseases through the air[19]; surface microbial analysis examines high-touch areas to assess pathogen persistence[20]. Together, these methods provide a multidimensional overview of pathogen circulation across environmental reservoirs, which improves the accuracy and reliability of early detection systems.
Therefore, by examining almost 300 published papers, the present manuscript aims to explore the role of WBE as an early warning system for future pandemics, emphasizing its applications, benefits, and limitations. Drawing on lessons from the COVID-19 pandemic, it discusses the potential of integrating WBE into global health surveillance frameworks to improve outbreak preparedness and response. By addressing technical, logistical, and infrastructural challenges, this study underscores the importance of proactive surveillance systems in mitigating the impact of emerging infectious diseases. As the global community faces evolving health threats, WBE stands out as a critical tool for safeguarding public health and strengthening global resilience against future pandemics.
The novelty of the present work is mainly associated with its integrative perspective, combining WBE with Aerobiology and surface microbiome analysis to establish a multidimensional environmental surveillance framework that enhances the reliability and cross-validation of early warning systems. Contrary to previous reviews, it repositions WBE as a proactive tool for pandemic preparedness, drawing on the lessons from COVID-19 and advocating for its expanded role in global health security. Furthermore, the present manuscript addresses the WBE implementation in LMICs, a frequently overlooked topic, by identifying technological and infrastructural challenges associated with these settings and proposing context-specific solutions. Moreover, the manuscript distinguishes itself from others by exploring the synergy between WBE and emerging technologies [e.g., biosensors, machine learning (ML), the Internet of Things - IoT, and metagenomics], presenting a forward-looking vision of digital and data-driven disease surveillance. Additionally, it highlights the role of coordinated global efforts, such as those led by the MetaSUB Consortium, in promoting standardized urban environmental monitoring and global collaboration.
The specific objectives of this manuscript are to explore the role of WBE as an early warning tool for emerging infectious diseases, to evaluate its integration with Aerobiology and surface monitoring, to assess its effectiveness through real-world case studies, particularly from the COVID-19 pandemic, to identify the main technical and operational challenges in diverse global contexts, to present technological innovations that enhance its application; to propose its inclusion in global health monitoring frameworks such as those by WHO and GISRS, and to discuss ethical, privacy, and governance considerations relevant to its implementation.
MATERIAL AND METHODS
For this review, manuscript selection followed a structured, stepwise approach to ensure scientific rigor and relevance [Figure 1]. The initial search retrieved 7,721 records from two major scientific databases: PubMed (6,521 articles) and Google Scholar (contributed 1,200). These searches employed carefully selected keywords, including “Wastewater-Based Epidemiology”, “Early Warning Systems”, “Pathogen Detection”, “Antimicrobial Resistance”, “Environmental Epidemiology”, and “Disease Outbreak Prediction”.
Figure 1. Schematic representation of the flowchart of the searched articles and the inclusion and exclusion criteria.
After removing duplicate entries, 6,521 unique records remained. These records were then subjected to an initial screening based on title and abstract review. Articles that did not relate to the review themes were excluded.
Subsequently, the full texts of 3,614 articles were assessed for eligibility. The assessment considered several factors: publication date, peer-review status, and scientific quality. Studies published prior to 2019, those that had not undergone peer review, and those lacking methodological robustness were excluded from further consideration. This filtering process led to the inclusion of 2,729 studies for qualitative analysis.
Only studies with accessible full-text documents were retained to refine the selection further for quantitative analysis. This final criterion narrowed the selection to 279 manuscripts. Throughout the process, preference was given to articles published in high-impact journals ranked within the Q1 and Q2 quartiles and to those demonstrating practical relevance through real-world applications or case study scenarios. After the final selection of eligible studies, all relevant data were systematically extracted and compiled into structured summary tables using Microsoft Excel. These tables were organized to categorize studies by themes such as methodology, pathogen types, geographic region, sample type, and key findings, allowing for a transparent and comparative synthesis of results across different surveillance approaches. Among the 279 manuscripts selected, only 117 references are cited in the manuscript, as these were the studies deemed most relevant for inclusion in the discussion and synthesis of results. The remaining articles contributed to the broader review process, helping to inform background understanding, context, and methodological comparisons, but were not directly cited.
This multi-layered selection strategy ensures the present review is grounded in high-quality, up-to-date literature. It offers a comprehensive and empirically supported overview of WBE and its role in addressing emerging public health challenges.
Although this methodology includes high-quality and relevant studies, it also presents potential biases that must be acknowledged. For instance, the review may be subject to publication bias, as studies with statistically significant or positive findings are more likely to be published in peer-reviewed journals. Additionally, the exclusion of non-English language articles could limit the scope of perspectives and data, particularly from regions where WBE has been applied but not reported in English. These constraints may have led to an underrepresentation of relevant research, especially from LMICs, and should be considered when interpreting the findings of this review.
While mindful of its limitations, this multi-layered selection strategy aimed to provide a robust, up-to-date, and comprehensive overview of WBE and its expanding role in public health surveillance and infectious disease management.
CURRENT LANDSCAPE OF PANDEMIC SURVEILLANCE AND EMERGING THREATS
Global context of pandemics
In recent years, the world has witnessed a concerning rise in the occurrence and impact of pandemics[21] due to international travel, global warming, or zoonotic transmission[22,23]. Noteworthy outbreaks include HIV, SARS-CoV, H1N1 influenza, MERS-CoV, Ebola, and SARS-CoV-2[24]. The rapid spread of infectious diseases and emerging threats not only underscores the vulnerability of global systems[25] but also exposes the weaknesses in preparedness and response mechanisms worldwide[23]. Pandemics not only pose severe risks to public health but also disrupt economies, overwhelm healthcare infrastructures, and strain societal functions[22].
The COVID-19 pandemic will forever remain a reminder of how quickly infectious diseases can spread in an interconnected world like ours[26]. In fact, this virus was able to establish itself in multiple countries around the globe before clinical cases were widely recognized, exacerbating the challenge of containment[27-29]. Together with coronaviruses, influenza viruses present high mutation rates coupled with rapid transmission. The two groups have caused pandemics throughout history and will continue to pose significant risks to global health security[30].
One of the main challenges in adequately managing pandemics lies in its early detection. Conventional surveillance systems often rely on the identification of clinical symptoms, meaning that the infection is already widespread by the time significant actions are taken[31]. Furthermore, asymptomatic carriers and delays in testing or reporting can mask the true spread coverage and, therefore, limit the effectiveness of any containment efforts[32]. The gap between the time of pathogens’ emergence and their detection allows diseases to proliferate, particularly in areas that are densely populated or present serious resource limitations[33].
All the challenges presented above highlight the need for proactive surveillance systems capable of identifying pathogens at the population-wide level before the first clinical cases emerge, thereby significantly improving preparedness and response efforts[1,34]. In this context, innovative approaches such as wastewater monitoring have demonstrated their potential as early warning tools[35,36]. Sewage water analysis allows the identification of traces of infectious agents, making it possible to detect disease activity in the population before significant clinical symptoms appear, allowing for timely public health interventions[17,37].
International organizations, mainly the World Health Organization (WHO), help shape, coordinate, and support global disease surveillance systems[38]. By unifying international efforts, establishing standardized protocols, and mobilizing resources, these organizations are essential actors in the early detection and control of infectious disease threats[39]. The rapid cross-border spread of pathogens, as exemplified by the COVID-19 pandemic, underscores the need for coordinated international responses and highlights the importance of globally harmonized surveillance mechanisms[39].
Within this context, integrating WBE into existing global surveillance frameworks is both timely and strategic[40,41]. As a non-invasive, cost-effective, and population-wide monitoring tool, WBE may be implemented as a complementary tool to traditional clinical surveillance by detecting early signals of pathogen circulation even in asymptomatic and pre-symptomatic infections[9]. Incorporating WBE into established programs [e.g., WHO’s Global Influenza Surveillance and Response System (GISRS) and the International Health Regulations (IHR) framework] significantly strengthens global preparedness for future outbreaks[38].
For this integration, the WHO and its partner organizations need to encourage the development of standardized guidelines for WBE implementation, promote capacity building in LMICs, and encourage data sharing through interoperable global platforms. Furthermore, collaborations among international consortia (e.g., MetaSUB) and national public health agencies enhance cross-border surveillance and provide actionable intelligence for timely interventions[20].
Ultimately, the institutional endorsement and leadership of international organizations, such as the WHO, scale WBE adoption and embed it as a core component of global health security. This integration enhances the early detection of infectious diseases and contributes to more equitable and resilient public health systems.
WBE as a surveillance tool
WBE, a concept developed in 2001 to either illegal drugs or drugs of abuse[42], had been previously used to trace antibiotic-resistant bacteria and genes in populations[43] and, more recently, has been adapted during the SARS-COVID 19 as an innovative approach for monitoring the spread of infectious diseases in human populations[44]. The analysis of samples collected from sewage systems for biological markers provides information on the presence of pathogens circulating within communities since infectious agents (e.g., viruses, bacteria, and parasites) are often shed in human waste through feces, urine, or other bodily fluids. These biological traces enter wastewater systems and can be analyzed to identify disease trends at a population level.
A notable advantage of WBE is its ability to detect pathogens before clinical cases are officially identified[13]. Infectious agents can be discharged by asymptomatic, pre-symptomatic, or mildly symptomatic individuals that often go unnoticed by traditional healthcare systems[31]. This discharge makes wastewater a highly informative medium for early detection, reflecting the health status of entire populations in almost real time. For instance, during the COVID-19 pandemic, several studies detected viral RNA in sewage days to weeks before clinical testing revealed an increase in the reported cases[45,46], making WBE a critical component of disease surveillance systems.
Moreover, WBE benefits extend far beyond early disease detection. This is a cost-effective strategy, covering large and diverse populations and providing a scalable solution for monitoring infectious diseases[47,48]. Contrary to individual testing, which is resource-intensive and logistically challenging, WBE enables broad population surveillance, significantly reducing both costs and efforts[49]. Additionally, WBE is non-invasive, relying on samples taken from sewage systems that are already in place, making it less intrusive and more acceptable to communities[50] [Figure 2].
Another pivotal WBE advantage is its ability to identify asymptomatic carriers[50]. Traditional surveillance methods often miss asymptomatic individuals who do not seek medical attention, yet these individuals play a significant role in disease transmission[51]. The capture and analysis of biological markers from the entire population, including undiagnosed or asymptomatic individuals, provides a more comprehensive understanding of disease spread and prevalence[52] [Figure 2].
A comparative analysis of WBE and other monitoring methods (e.g., clinical surveillance and aerobiological sampling) is required to understand the strengths and limitations of each approach under various public health contexts. While clinical monitoring remains the gold standard for individual diagnosis and case confirmation, it is resource-intensive, dependent on healthcare access, and often delayed due to testing and reporting lags. In contrast, WBE offers a cost-effective and non-invasive tool for community-level surveillance, capable of detecting pathogens shed by symptomatic and asymptomatic individuals, often before clinical cases are reported. On the other hand, Aerobiology enables real-time detection of airborne pathogens, particularly in enclosed or high-risk environments, but environmental conditions and spatial variability influence its effectiveness. Evaluating these methods regarding cost, efficiency, and accuracy reveals that they are not mutually exclusive but complementary. Their integration into a cohesive surveillance framework can enhance early warning capabilities, improve resource allocation, and support more timely and targeted public health responses [Table 1].
Comparative analysis of wastewater-based epidemiology (WBE), clinical monitoring, and Aerobiology
| Criteria | WBE | Clinical monitoring | Aerobiology |
| Cost | Low to moderate per capita; scalable; cost-effective for large populations | High per case (testing, diagnostics, reporting infrastructure) | Moderate; requires specialized sensors and frequent maintenance |
| Efficiency | High for community-level trends; early detection of asymptomatic cases | Individual-level diagnosis; delayed signal for population trends | Real-time detection in high-risk areas; good for targeted zones |
| Accuracy | High for pooled data; limited individual resolution; affected by dilution | High for confirmed cases; gold standard for individual diagnosis | Variable; influenced by air flow, weather, and sampling location |
| Timeliness | Early signal before clinical reports; weekly or daily sampling possible | Often delayed by testing/reporting lag; depends on healthcare access | Near real-time with automated sensors; location-specific |
| Scalability | Highly scalable; one sample represents thousands of people | Limited by testing infrastructure and workforce | Scalable in targeted high-risk environments (e.g., hospitals) |
| Privacy risk | Low; aggregate, anonymized data | Moderate to high; involves personal data and test results | Low; environmental sampling without direct human identifiers |
| Best use cases | Early outbreak detection; community-level surveillance | Confirming and treating individual cases | Monitoring airborne transmission risks in closed environments |
Despite the numerous technical advantages, the increasing adoption of WBE raises critical ethical considerations, mainly concerning data privacy and the use of community-level health information. Although WBE does not directly identify individuals, its application in small or localized populations (e.g., neighborhoods, dormitories, correctional facilities) potentially leads to stigmatization or unintended community profiling if results are misinterpreted or disclosed without adequate context.
Nevertheless, in the case of rapidly evolving infectious threats and public health emergencies, collective health and safety protection must take precedence over individual privacy concerns[53]. Early detection of pathogens enables timely interventions that help prevent widespread transmission, protect vulnerable populations, and reduce the burden on healthcare systems[54,55]. Consequently, WBE constitutes a proactive tool prioritizing communities’ well-being, especially when delayed action results in significant morbidity or mortality.
Transparency, responsible data governance, and community engagement ensure that WBE respects human rights and fulfills its critical role in disease prevention. Finally, safeguarding public health through early and collective action is viewed as a scientific imperative and an ethical responsibility[56,57].
Aerobiology: a complementary approach to validate WBE results
Aerobiology is the study of airborne dynamics of microorganisms such as viruses, bacteria, and fungal spores, aiming to understand their source and transmission through the air[19]. Due to their ability to spread rapidly through aerosols and respiratory droplets, airborne pathogens, particularly respiratory viruses, constitute a significant threat[58].
While WBE focuses on detecting pathogens shed in human waste and present in sewage systems, Aerobiology identifies airborne pathogens and their transmission routes[19]. This complementary approach becomes particularly important to trace respiratory-transmissible diseases, such as those caused by SARS-CoV-2 or influenza viruses. These viruses are not only shed in wastewater but also through exhalation, coughing, and other respiratory activities. Therefore, the analysis of airborne pathogens validates the presence of the same pathogens in wastewater, providing an additional layer of evidence confirming infection trends within a given population.
The combination of WBE and Aerobiology creates a more robust and comprehensive surveillance strategy. While WBE provides an early indication of infection levels within a given community, often before the first clinical symptoms emerge[18], Aerobiology pinpoints airborne transmission hotspots and environmental conditions that facilitate pathogen dissemination[45]. Together, these two methods enable public health authorities to detect, validate, and respond to emerging outbreaks with higher accuracy and confidence [Figure 2].
For diseases that primarily transmit through the respiratory route, Aerobiology is particularly valuable. For instance, during the COVID-19 pandemic, studies identified viral particles in both indoor and outdoor air[59,60], reinforcing WBE findings of community-level viral circulation. By integrating airborne monitoring with sewage analysis, it is possible to cross-reference data and strengthen predictions of infection trends. This constructive collaboration ensures a clearer understanding of disease dynamics and transmission pathways, which is critical for implementing targeted interventions.
Technological advancements have recently introduced biosensors and real-time monitoring systems as innovative tools to enhance pathogen detection and monitoring in air and wastewater surveillance[61,62]. In Aerobiology, biosensors offer rapid, sensitive, and on-site detection of airborne microorganisms (e.g., fungi, viruses and bacteria)[63,64]. These devices continuously sample ambient air in high-risk environments (e.g., hospitals, airports, or public transit hubs), detecting pathogen-specific biomarkers in real time[63]. Integrating biosensors into existing surveillance networks provides public health authorities with immediate access to actionable data, facilitating early responses to potential outbreaks[63]. Additionally, when used alongside WBE, biosensor-based aerobiological monitoring provides cross-validation of infection trends and strengthens early warning systems. Implementing automated sampling devices and IoT-enabled biosensors marks an evolution toward continuous, real-time environmental surveillance and bridges the gap between laboratory analysis and field-based disease detection[9].
Analysis of microorganisms on highly touched surfaces
Frequently touched surfaces from urban environments, such as handrails, ticketing kiosks, and turnstiles in metro systems, harbor and transfer microbial communities, including potentially harmful pathogens[65]. These high-touch areas function as reservoirs for microorganisms introduced through human activity, environmental exposure, and aerosol deposition[66]. Therefore, the analysis of the microbial composition of these surfaces provides essential information into pathogen dynamics and their interactions with human behavior in dense, high-traffic spaces.
Surface sampling enables the identification of microbial communities on these high-touch surfaces, facilitating the detection of potential pathogens and AMR genes, while also tracking changes in microbial diversity influenced by public activity and environmental factors[67]. The data collected in these studies reveal how human touchpoints contribute to disease transmission and highlight areas where interventions, such as cleaning protocols or the redesign of high-touch zones, can reduce transmission risks[68-70].
Surface microbial analysis incorporates an additional layer of insight into urban pathogen monitoring in broader surveillance systems[71]. While WBE provides a population-level overview of pathogen prevalence[13], and Aerobiology captures airborne particles that are responsible for disease transmission[19], surface analysis focuses on contact-based transmission routes[68]. The interaction between these three methodologies provides a multidimensional overview of pathogen behavior across urban environments, which enhances detection accuracy and the ability to respond to emerging threats [Figure 2].
Despite its value in tracking pathogen transmission, surface sampling may provide false positive results that arise from environmental contamination, residual non-viable genetic material, or improper sample handling, leading to the detection of no longer infectious or clinically relevant organisms[72-74]. To avoid false positives, it is essential to establish standardized protocols that include rigorous surface sterilization controls, validated swabbing techniques, and the implementation of nucleic acid viability assays to distinguish between live and dead microorganisms[75,76]. Additionally, confirmatory testing using quantitative PCR (qPCR) and next-generation sequencing (NGS) improves result specificity and reliability[77,78]. These practices ensure surface surveillance data accurately reflect active microbial threats and support meaningful public health decisions.
Global efforts and the role of the MetaSUB consortium
The MetaSUB Consortium (metagenomics and metadesign of subways and urban biomes), a global initiative, focuses on the surveillance of microbial communities across urban environments. Based on an interdisciplinary approach, MetaSUB integrates monitoring of multiple environmental compartments (e.g., air, water, surfaces, and wastewater) to provide a comprehensive understanding of microbial dynamics in densely populated areas. With the study of these interconnected ecosystems, the consortium aims to identify emerging pathogens and track their dissemination and transmission routes in urban settings[20].
These high-density urban environments serve as potential hubs for pathogen transmission, providing fertile ground for close contact between disease vectors (e.g., Aedes mosquitoes) and the population[79] but also facilitate horizontal gene transfer (HGT) events between different microorganism species that colonize both humans and animals[80]. In its integrative approach, MetaSUB has mapped and analyzed metagenomes from subways, public spaces, and wastewater systems, providing an understanding of the interaction between microbes and these spaces[81]. The consortium uses innovative technologies (e.g., metagenomic sequencing and ML) to identify genetic material from pathogens and other microorganisms, contributing to the early detection of infectious agents before significant outbreaks occur[81].
One of the consortium’s strengths lies in its centralized model, where all sampling protocols, materials, and reagents are uniformly provided by the Manson Lab at Cornell University. This centralization ensures methodological consistency and comparability of samples collected from vastly different environments across the globe. Additionally, sequencing is also centralized and conducted at the Manson Lab, which guarantees uniform data quality and minimizes technical variability.
During the COVID-19 pandemic, MetaSUB played a significant role in tracking the spread of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) virus and its variants in several cities across the world[20]. During the lockdown, and with the acknowledgment of public health authorities, consortium’s members collected samples from surfaces, wastewater, and air in urban transit systems and public spaces that successfully identified viral traces and provided critical data on the movement and persistence of the virus[20]. These efforts highlighted the potential of integrating environmental surveillance with public health systems to monitor and contain outbreaks more effectively[82]. The MetaSUB’s findings underscored the importance of a global, coordinated response to emerging infectious threats, as the consortium facilitated data sharing across cities and countries to strengthen pandemic readiness[83].
The MetaSUB Consortium’s interdisciplinary nature combines expertise from several research fields, such as microbiology, genomics, epidemiology, and environmental science, which enhances the global knowledge on urban microbial ecosystems[20,25]. By unifying data from different environmental sources, MetaSUB has demonstrated the value of a holistic surveillance strategy that not only enables the early detection of pathogens but also provides insights into AMR, microbial evolution, and the dynamics of disease transmission in urban populations[20].
WBE: STRENGTHENING OUR EARLY WARNING SYSTEMS
Principles of WBE
WBE is based on the principle that pathogens are shed in human waste and can be detected within sewage systems[9]. Infected individuals - whether symptomatic, asymptomatic, or pre-symptomatic - excrete the molecular signatures of these pathogens through their body fluids (e.g., urine, feces) that enter wastewater streams, which can be analyzed to assess the health status of the entire population[16].
WBE to detect pathogens before widespread clinical symptoms emerge makes it a powerful early warning system. For instance, during the COVID-19 pandemic, traces of SARS-CoV-2 RNA were identified in sewage weeks before increases in confirmed cases were observed through clinical testing[84], highlighting the sensitivity of wastewater monitoring in capturing the real-time presence of pathogens circulating in communities. Likewise, bacteria such as Salmonella or protozoa such as Giardia or Cryptosporidium can also be detected in wastewater, providing a broader scope for monitoring various infectious diseases[85] [Figure 3].
Wastewater surveillance involves sequential, well-defined steps, such as sampling, analysis, and quantification. During sampling, wastewater is collected from specific sites (e.g., sewage treatment plants, industrial zones) or targeted areas (e.g., neighborhoods, university campuses). Depending on the study’s objective, samples may be collected as 24-hour composite samples or as grab samples for immediate analysis[86]. Among the various sampling techniques, 24-hour composite sampling constitutes the most effective method compared to grab sampling since it considers the fluctuations in wastewater composition throughout the day. Human activities significantly affect water flow and content, leading to considerable variations in microbial load, chemical composition, and hydraulic flow at different times. By integrating aliquots collected at regular intervals over 24 h, composite samples provide a more accurate picture of average pathogen concentrations. This method significantly reduces the risk of misestimating pathogen loads, possibly if a grab sample is collected during an unusual spike or drop in wastewater flow. Therefore, adopting 24-hour composite sampling significantly improves WBE data’s accuracy, consistency, and interpretability, making it an invaluable tool for effective surveillance and trend analysis[87]. Once collected, samples are processed to isolate and identify pathogen-specific markers (e.g., viral RNA, bacterial DNA, or protozoan genomes) using advanced techniques (e.g., RT-qPCR) that detect and quantify viral or microbial genetic material with high sensitivity and accuracy[88]. Additionally, more sophisticated methods such as NGS can be employed to analyze the full genetic composition of pathogens present in sewage. This technique not only confirms the presence of specific pathogens but also enables the identification of mutations, new variants, or AMR genes[89].
Pathogen markers can be lost or degraded in wastewater due to the complexity of this matrix. Several factors influence the persistence and detectability of microbial signatures in sewage (e.g., dilution effects from stormwater or industrial discharge, presence of disinfectants and organic compounds, temperature and pH, enzymatic activity from naturally occurring microbes, and microbial competition). Pathogen markers may also be lost due to their adsorption to solid particles, which can complicate their extraction and subsequent detection[66,90].
Detection limits vary depending on the type of pathogen, the matrix, and the analytical method used. For example, in the case of SARS-CoV-2, optimized RT-qPCR protocols have achieved detection limits as low as 1-10 genome copies·mL-1 of wastewater[91]. One study demonstrated that SARS-CoV-2 RNA could be detected at concentrations as low as 103 copies·L-1[92], offering public health authorities up to a seven-day lead time before increases in clinical cases were reported. For norovirus, another RNA virus commonly found in wastewater, detection limits range between 102 and 103 genome copies·L-1[93,94]. Bacterial pathogens, such as Salmonella enterica, are generally detected at levels around 104 CFU·mL-1 using conventional PCR, although this threshold can be lowered with qPCR and sample enrichment[95].
To mitigate these challenges and improve detection sensitivity, several strategies have been adopted. Concentration techniques, such as polyethylene glycol (PEG) precipitation or ultrafiltration, are commonly used to enrich target nucleic acids. Digital PCR (dPCR) is increasingly favored for its enhanced sensitivity and ability to quantify low-abundance targets, even in the presence of inhibitors. Pre-treatment steps, including filtration and chemical purification, can also reduce matrix interference. Finally, the adoption of standardized protocols [e.g., recommended by the Centres for Disease Control and Prevention (CDC) or the WHO] is essential for ensuring the reproducibility and reliability of results across different laboratories and regions[96-98].
Sewage surveillance during COVID-19
The COVID-19 pandemic reinforced the value of WBE as a practical and efficient surveillance tool for monitoring the spread of SARS-CoV-2. Worldwide sewage surveillance campaigns provided valuable insights into infection dynamics, serving as an early warning system that complemented clinical testing and public health interventions[99].
Several studies from different world regions highlight the success of wastewater monitoring during the pandemic. For instance, in Spain, the Netherlands, and Belgium, SARS-CoV-2 RNA was detected in wastewater samples weeks before the first confirmed cases of COVID-19 in the population[43,100]. In the United States, a correlation was observed between viral RNA concentrations in wastewater and clinical cases and hospital admissions, providing advance notice of 0-8 days[101], enabling the anticipation of upcoming case spikes, optimization of testing strategies, and implementation of preemptive measures to reduce community transmission. In England, sewage analysis facilitated the identification of co-circulating virus variants and the monitoring of changes in viral predominance over time[102], while in other locations, it also enabled the detection of novel mutations[43], making it a powerful tool for tracking viral evolution [Table 2].
Summary of key studies utilizing WBE during the COVID-19 pandemic
| Country/region | Sampling site | Lead time (WBE vs. clinical cases) | Public health outcome | Ref. |
| Spain | Wastewater treatment plant | 7-14 days | Early warning → intensified testing | [100] |
| USA (Connecticut) | Municipal sewage network | 0-8 days | Forecasted hospitalizations | [101] |
| the Netherlands | National wastewater system | ~1 week | Supported lockdown decisions | [43] |
| England | Sewage from metro areas | Tracked variant shifts | Guided variant monitoring and policy change | [102] |
Strengths and limitations of sewage monitoring
One of the main strengths of sewage monitoring is its cost-effectiveness. In contrast with individual clinical testing that requires significant resources, skilled personnel, and time, wastewater sampling allows for the near real-time monitoring of large populations using a single sample, enabling health authorities to assess the health status of entire communities without the need for extensive individual testing[103,104].
Secondly, WBE is linked to its non-invasive nature, since the process relies on the collection of sewage samples, a byproduct of human activity, that does not require direct interaction with individuals, making it an acceptable and practical tool for population-level surveillance, especially in settings where conventional testing may face resistance or logistical challenges[13,54].
Another WBE advantage is its high scalability, as it enables the surveillance of large populations by analyzing data from thousands or even millions of individuals at once, providing a comprehensive overview of pathogen prevalence and trends across entire cities or regions[36].
In addition to monitoring pathogens, wastewater often contains AMR genes, including those carried on plasmids, which can persist even in treated effluents[104,105]. This aspect highlights the potential of WBE for tracking the spread of resistance and raises concerns about wastewater systems acting as reservoirs and dissemination pathways for AMR, thereby contributing to the evolution and environmental spread of resistant microorganisms.
Finally, WBE’s ability to detect asymptomatic infections is one of its main strengths. Individuals infected with pathogens begin shedding them in their body fluids early in the infection cycle, even in asymptomatic and pre-symptomatic cases, providing a more comprehensive view of community disease burden than clinical surveillance alone[106].
Despite its strengths, WBE also presents limitations. One of them is the variability in sewage systems across different regions [Figure 4], influenced by factors such as population density, wastewater collection efficiency, and sewage treatment infrastructure. For instance, informal settlements or rural areas with limited sewage infrastructure may not be adequately monitored, leading to gaps in surveillance[99].
Figure 4. Opportunities and challenges of wastewater-based epidemiology (WBE) implementation in high-income and resource-limited settings. RT-qPCR: Rerverse transcriptase quantitative polymerase chain reaction.
Another limitation is associated with sample degradation. Over time, pathogens and their genetic material are degraded due to the influence of temperature, pH, and chemical composition of the sewage. These factors - which can not only reduce the sensitivity of detection methods but also compromise the accuracy of results, especially in warm climates or when samples are not promptly processed - must be carefully considered when using a WBE approach for pandemic monitoring[107].
Finally, the implementation of sewage monitoring requires advanced infrastructure and skilled technical expertise. Accurate WBE analysis involves sophisticated techniques such as RT-qPCR or NGS, which require specialized laboratories, trained personnel, and financial investment. In resource-limited settings, such as LMICs, these requirements constitute a major barrier to the widespread adoption of WBE as an early warning system for future pandemics[84].
An important limitation of WBE is that not all pathogens are excreted in urine or feces, and for those that are, shedding may occur only intermittently or during a limited window of infection, which can hinder detection and affect the accuracy of prevalence estimates. For instance, pathogens such as Mycobacterium tuberculosis (primarily transmitted via respiratory droplets), Treponema pallidum (the causative agent of syphilis), and Plasmodium species (responsible for malaria) are typically not excreted in sufficient quantities in wastewater, limiting their detectability through this surveillance approach.
WBE presents excellent potential in LMICs due to its cost-effectiveness and capacity to monitor large populations with minimal infrastructure and limited access to clinical testing[9]. However, WBE implementation in these countries also presents several challenges, such as limited access to centralized sewage systems, lack of trained personnel, insufficient laboratory capacity, and inadequate funding for advanced molecular diagnostics (e.g., RT-qPCR or sequencing), which hinder its deployment[108]. Furthermore, logistical constraints in sample transport, storage, and timely analysis often reduce the reliability of results. Simplified protocols, portable testing platforms, and international infrastructure development and capacity-building support are essential to overcome these barriers[9]. Strengthening partnerships with global health organizations can also facilitate the integration of WBE into existing public health strategies in LMICs[39,109].
Strengthening WBE with Aerobiology
Integrating WBE with Aerobiology can significantly enhance its effectiveness. Aerobiology focuses on the study of airborne microorganisms (e.g., viruses, bacteria, and fungal spores) that persist in the air, particularly in enclosed or poorly ventilated spaces where the risk of transmission is higher. By collecting and analyzing air samples, aerobiological methods enable the detection of aerosolized pathogens, offering valuable insights into respiratory transmission within populations[110].
The combination of WBE and Aerobiology allows for cross-validation of epidemiological data. For instance, if the detection of SARS-CoV-2 RNA in sewage signals a potential outbreak, airborne monitoring confirms its presence in areas of high human activity, such as public transportation, schools, or healthcare facilities. This dual surveillance approach increases the results’ reliability and pinpoints specific environments requiring specific intervention measures, such as improved ventilation or localized restrictions[111]. Moreover, this integrative approach also provides a more comprehensive understanding of pathogen circulation. WBE reflects the overall infection burden in each population, including asymptomatic and pre-symptomatic individuals, while Aerobiology captures pathogens’ airborne spread, a critical aspect of diseases transmitted via the respiratory route[9]. Therefore, when combined, these methods offer a holistic view of pathogens dynamics through populations and environments, enabling better predictions of disease behavior and more informed public health responses.
FROM SEWAGE TO STRATEGY: BUILDING RESILIENCE AGAINST FUTURE PANDEMICS
Potential pathogens for wastewater surveillance
Wastewater contains traces of pathogens shed in body fluids from infected individuals that can often be detected before the record of the first clinical cases in the population. This early detection window is particularly valuable for rapidly spreading or asymptomatic infections, where traditional monitoring systems might fail to provide timely warnings[112-114].
Among viral pathogens, influenza viruses and norovirus are examples of agents that can be monitored through wastewater surveillance [Table 3]. Influenza viruses, which cause seasonal epidemics and present the potential to trigger pandemics, are shed in human waste and detectable in sewage samples. Similarly, norovirus, responsible for gastrointestinal infections, can be identified in wastewater before significant cases are reported in healthcare settings[115].
Representative pathogens detectable through WBE and their relevance for surveillance
| Pathogen | Type | Transmission route | Surveillance relevance |
| SARS-CoV-2 | Virus | Respiratory | Early pandemic detection and monitoring |
| Norovirus | Virus | Fecal-oral | Gastroenteritis outbreak prediction |
| Salmonella spp. | Bacterium | Foodborne | Foodborne outbreak tracing |
| E. coli (STEC) | Bacterium | Food/waterborne | Indicator of fecal contamination |
| Giardia/Cryptosporidium | Protozoa | Waterborne | Detection in areas with poor sanitation |
| AMR genes | Genetic markers | Varies | Monitoring of AMR |
Bacterial pathogens and AMR genetic markers are other key targets for wastewater surveillance. For instance, Salmonella and Escherichia coli, responsible for foodborne illnesses, can be detected and tracked in wastewater to identify infection hotspots. Additionally, the presence of AMR genes in sewage provides valuable information about the spread of resistant bacterial strains within communities[116-118] [Table 3]. Considering the actual growing threat of AMR, monitoring AMR markers in wastewater samples may assist public health officials in anticipating emerging challenges and designing targeted interventions during disease outbreaks [Table 3][9].
Parasitic infections, such as those caused by Giardia lamblia and Cryptosporidium, responsible for waterborne illnesses, also shed detectable markers into sewage systems during winter and spring
Recent advancements, particularly in untargeted metagenomic sequencing and bioinformatics, have enabled the detection of novel pathogens and previously unknown genetic signatures (i.e., emerging pathogens) in wastewater samples, expanding WBE’s utility beyond known targets[121]. However, these techniques also present some challenges. Rapid identification of novel pathogens demands sophisticated laboratory infrastructure, skilled personnel, and high-throughput sequencing platforms, which may not be available in all regions (e.g., LMICs)[122]. Moreover, interpreting novel sequences without reference genomes or clinical correlation may result in uncertainty or false alarms[123]. Timeliness is another challenge, as sample collection, transport, and processing can delay actionable insights, mainly in decentralized or resource-limited settings[124].
The effectiveness of WBE varies considerably according to environmental conditions and infrastructural contexts. Meteorological conditions (e.g., temperature, rainfall, and sewage system design) directly influence sample quality and pathogen detectability[125]. High temperatures promote nucleic acid degradation, and heavy rainfall or stormwater infiltration dilutes viral or bacterial concentrations, reducing sensitivity[126,127]. Arid environments correspond to more concentrated samples and pose challenges for sample flow and collection frequency[128,129]. Sample variability is also associated with community behavior, population density, and diurnal sewage flow, resulting in inconsistent pathogen loads[130,131], highlighting the need for standardized sampling protocols, normalization strategies (e.g., using fecal indicators or population biomarkers), and routine sampling schedules. Infrastructure limitations (e.g., the absence of centralized sewer systems or reliance on septic tanks) also restrict WBE reach in low-resource or rural areas[132-134], requiring adaptations such as targeted site sampling or the use of decentralized collection systems[108,135].
The future of WBE is closely related to advancements in detection technologies and data analysis methodologies that enhance the sensitivity and specificity of surveillance efforts significantly[39]. Emerging tools, such as digital droplet PCR (ddPCR), NGS, and CRISPR-based diagnostics, ensure accurate pathogen quantification and identification, even for low-abundance targets, novel genetic variants, and AMRs[136-138]. These technologies reduce the probability of false positives and enable the simultaneous detection of multiple pathogens or resistance genes. Additionally, ML and predictive analytics easily interpret complex datasets, identify trends, and forecast outbreaks more precisely, due to the incorporation of temporal, geographic, and environmental variables to enhance the contextual understanding of disease dynamics[139-141]. Furthermore, cloud-based platforms and real-time data dashboards facilitate rapid communication between laboratories, public health agencies, and decision-makers, enabling faster and more coordinated responses[142-144]. As these technologies become more accessible and cost-effective, they promise to expand WBE’s reach, accuracy, and public health value as a cornerstone of global surveillance infrastructure[145,146] (for further details, see Section “Technological advancements”).
As previously referred, the accuracy and reliability of wastewater surveillance can be significantly enhanced by combining WBE with Aerobiology. Integrating data from sewage samples with results from airborne surveillance enables the validation of WBE findings and allows for more precise detection and tracking of infectious agents[111]. This combined approach is remarkably beneficial for pathogens with dual transmission routes, such as SARS-CoV-2, which can be detected in wastewater and the air.
Integration into global health surveillance systems
The integration of WBE into existing global health surveillance systems can revolutionize how public health authorities detect and respond to emerging infectious diseases[132]. By providing real-time, population-centered data on pathogen circulation, WBE serves as a complement to traditional clinical monitoring while enhancing the capacity for early warning and containment[9].
One notable example of global collaboration in this field is the MetaSUB Consortium, which, through its innovative methodologies and interdisciplinary approach, has significantly contributed to tracking pathogens and microbial communities across diverse urban environments. This consortium integrates sampling from multiple sources (e.g., air, high-touch surfaces, and wastewater) to build comprehensive snapshots of pathogen distribution from more than sixty cities around the world. By employing advanced techniques such as metagenomics, MetaSUB has successfully identified emerging threats, including AMR genes and viral pathogens such as SARS-CoV-2. The work developed by the consortium underscores the importance of global initiatives in producing valuable, actionable data for disease surveillance and preparedness[20,64].
Moreover, organizations such as the WHO and national public health agencies such as the CDC, which already play a role in disease monitoring and outbreak response, should incorporate WBE as an early detection tool for outbreaks. This would complement traditional surveillance systems, especially in areas with social vulnerabilities and poor sanitation, where pollution is a concern[13,147]. For instance, the detection of viral or bacterial pathogens in wastewater can trigger targeted clinical testing, resource allocation, and public health interventions in the affected areas.
An advantage of incorporating wastewater surveillance into global frameworks is its scalability and adaptability[36]. Wastewater systems are present in urban, suburban, and rural areas, making WBE an accessible option for monitoring diverse populations. Moreover, global networks such as the WHO’s GISRS should incorporate WBE to enhance the detection of influenza and other respiratory disease outbreaks. The harmonization of WBE methodologies and data-sharing practices will provide an additional layer of information on pathogen trends on a worldwide scale.
Finally, integrating WBE data with other surveillance tools, such as Aerobiology and clinical reporting systems, enables the development of a more robust and comprehensive approach to monitoring public health threats. The cross-validation of wastewater findings with other sources improves accuracy, reduces false positives, and provides a clearer understanding of disease dynamics[148,149].
Technological advancements
The continuous WBE development has been driven by significant technological advancements that improved pathogen detection, analysis, and predictive modeling. These innovations transformed WBE into a powerful, scalable tool for real-time disease surveillance, valuable in pandemic preparedness and public health monitoring[150,151].
Advancements in PCR molecular biology techniques, such as quantitative RT-qPCR, have significantly increased the sensitivity and specificity of pathogen detection in wastewater samples. RT-qPCR, which remains a cornerstone of wastewater surveillance, allows for the quantification of viral or bacterial concentrations in sewage samples[88,152]. Moreover, improvements in assay design, such as one-step single-tube nested qRT-PCR (OSN-qRT-PCR) or RT-droplet digital PCR (RT-ddPCR), have made it possible to target specific genes of interest, ensuring precise detection and tracking of pathogens[153,154].
More recently, advances in metagenomics have revolutionized the ability to analyze complex biological samples collected from sewage systems. Metagenomic sequencing allows for the comprehensive identification of pathogens present in wastewater, including viruses, bacteria, and AMR genes, and identifies their potential for horizontal gene transfer[155-157]. Sequencing the entire composition of the genetic material within a sample enables the detection of known and emerging pathogens, offering a broad-spectrum approach to monitoring infectious diseases[89,158]. This technique has been particularly valuable for identifying variants of concern, such as SARS-CoV-2 mutations, during the COVID-19 pandemic[43,102].
Another innovation is the integration of artificial intelligence (AI) and ML for predictive modeling[159]. AI-driven approaches, such as artificial neural networks (ANN), fuzzy logic algorithms, and long short-term memory (LSTM) models, can be applied to big data compiled from wastewater metagenomics to identify trends, predict infection surges, and highlight areas at higher risk of outbreaks[160,161]. By combining historical data with real-time inputs, predictive models can be developed to anticipate disease dissemination and provide health authorities with critical lead time to implement control measures[162-164]. These AI-driven systems can detect patterns that might otherwise go unnoticed, thereby improving the overall accuracy and efficiency of wastewater surveillance[165]. During the COVID-19 outbreak, ML predictions, particularly time-series models such as LSTM, outperformed non-time-series models in forecasting cases across diverse sewersheds[166]. Additionally, these methods have been used to study antibiotic resistance in water and wastewater, with shallow learning techniques proving more prevalent than deep learning approaches[167].
The development of real-time analysis systems, such as biosensors, has further strengthened the role of WBE in global health surveillance[61]. These systems target specific molecules such as viral RNA, bacterial DNA, and AMR genes. For example, RNA-based electrochemical biosensors have been successfully applied to detect SARS-CoV-2 in wastewater by targeting conserved regions of the viral N gene, allowing for rapid and sensitive on-site surveillance[168-170]. Automated sampling devices and portable testing equipment enable faster sample collection and on-site analysis, reducing the time between sample acquisition and result generation[171,172]. The integration of cloud computing, the Internet of Medical Things (IoMT), and big data analytics has further enabled rapid collection, analysis, and sharing of health data[173-175], resulting in real-time monitoring of patient conditions, reducing healthcare costs, and improving diagnostic accuracy[176,177].
Data-sharing platforms have also facilitated collaboration and global monitoring. Platforms such as the Global Sewage Surveillance Network and initiatives driven by organizations such as the MetaSUB Consortium provide centralized repositories for WBE data. These systems allow researchers and health officials to upload, access, and compare pathogen data across worldwide regions. The aggregation of sewage surveillance data from multiple countries within these platforms contributes to a more comprehensive understanding of global disease trends and improves pandemic preparedness.
FROM THEORY TO PRACTICE: CASE STUDIES HIGHLIGHTING WASTEWATER SURVEILLANCE APPLICATIONS
COVID-19 and beyond
The COVID-19 pandemic highlighted the value of WBE as a reliable tool for the detection of pathogens’ presence and dissemination among communities. The analysis of SARS-CoV-2 RNA from wastewater enables the monitoring of infection trends and anticipates infection spikes before they are reflected in clinical testing data. This early detection of viral RNA often preceded a rise in reported cases by days or weeks, providing public health authorities with lead time to implement preventive measures[101,178]. In addition to WBE, Aerobiology has also been used to monitor airborne transmission of SARS-CoV-2, particularly in indoor environments (e.g., hospitals, transportation hubs, and public spaces)[179]. The detection of viral aerosols complemented wastewater surveillance data, validated findings, and pinpointed high-risk areas where transmission was most likely to occur[180]. The success observed with this integrative approach during COVID-19 was further stressed by global initiatives, such as the MetaSUB Consortium, which conducted large-scale microbial surveillance across cities worldwide, sampling air, surfaces, and wastewater to map the spread of SARS-CoV-2 and other pathogens. The integration of data from multiple environmental sources provided a holistic overview of pathogen dynamics in urban settings, identified hotspots of viral activity, and monitored the variant’s evolution[20].
Beyond COVID-19, these refined tools and methods should address future health threats. WBE can monitor a wide range of pathogens, including influenza viruses, AMR genes, and gastrointestinal infections (e.g., norovirus). Additionally, Aerobiology will continue to play a complementary role to WBE, tracking respiratory-transmissible agents, particularly in densely populated environments.
Lessons learned from COVID-19
The COVID-19 pandemic provided insights into the importance of preparedness and highlighted the transformative role of WBE in global health resilience. While public health systems faced unprecedented challenges, WBE emerged as a valuable surveillance tool, offering SARS-CoV-2 early detection, and enabling the design and implementation of proactive measures to mitigate its dissemination. These lessons accentuate the need for integrating wastewater monitoring into routine public health strategies to improve preparedness for future pandemics[13].
One of the most significant takeaways from COVID-19 is the importance of reliable early warning systems for outbreak detection. Traditional surveillance methods, such as clinical testing and hospital reporting, often lag behind the actual disease dissemination, particularly during the outbreak stages. Contrarily, WBE has proven effective in providing early signals of SARS-CoV-2 circulation within communities - days to weeks before case spikes. The detection of viral RNA in wastewaters allows public health authorities to anticipate infection surges, allocate resources efficiently, and implement targeted interventions such as increased testing and localized restrictions[13,84].
Secondly, the pandemic demonstrated the ability of WBE to monitor asymptomatic and pre-symptomatic infections. Since individuals shed viral material in their body fluids regardless of symptom status, wastewater surveillance offers a more comprehensive reflection of community-level infections compared to more traditional clinical testing. This capability proved particularly valuable in areas where access to testing was limited or underutilized, allowing health officials to identify “hidden” transmission and take pre-emptive action[13,150].
Another key lesson was the adaptability and scalability of WBE. During the pandemic, researchers and public health agencies worldwide quickly established wastewater monitoring networks, demonstrating how the method could be implemented across diverse settings, from urban to rural communities. This rapid deployment highlighted WBE’s potential to provide timely, cost-effective data that can guide public health decision making at local, national, and global levels[46,47,180].
The role of preparedness extends beyond surveillance and should integrate complementary tools. COVID-19 highlighted the importance of cross-validation between different protocols and sources, where sewage surveillance data were strengthened by airborne pathogen monitoring, particularly for respiratory-transmitted diseases. The combined use of these methods will enhance the accuracy of detection and improve the understanding of the dynamics of viral spread, further emphasizing the need for integrated surveillance frameworks[181].
Finally, the COVID-19 pandemic also served as a wakening call for the global community, emphasizing the need for investments in proactive health monitoring systems. WBE’s success during the pandemic demonstrated that early, population-based surveillance plays a significant role in improving health resilience. Detecting outbreaks before clinical cases are reported by wastewater monitoring enables public health systems to act instantly and reduce the impact of infectious diseases on communities[147,182].
Applications beyond pandemics
While WBE has proven its value in the detection and monitorization of pathogens during the COVID-19 pandemic, its applications extend far beyond outbreak surveillance. This approach offers a versatile tool for tracking a wide range of public health and environmental threats, including AMR, environmental pollutants, and the emergence of novel pathogens[9,11].
One of the most pressing global health concerns nowadays is the rising trends of AMR. Antibiotic-resistant bacteria and the genes responsible for its resistance are shed in human waste, being transported into sewage systems. Wastewater monitoring detects and quantifies AMR genes and resistant bacterial strains presenting these genes, constituting an early signal of AMR hotspots within communities. Tracking these markers over time allows WBE to identify trends in antibiotic misuse, assess the dissemination of resistant pathogens, and implement strategies for reducing AMR risks[183]. For instance, hospitals and healthcare facilities often function as reservoirs for resistant bacteria. Wastewater surveillance constitutes an effective tool to monitor healthcare-associated infections and evaluate antibiotic stewardship programs’ success, helping to understand the dynamics of AMR emergence, dissemination, and support global initiatives to combat antibiotic resistance[66].
Beyond infectious diseases, WBE has also been employed to monitor hazardous environmental pollutants associated with human and ecological health risks. While industrial chemicals, pharmaceuticals, pesticides, and heavy metals can enter wastewater systems through human activity and industrial processes, wastewater monitoring detects and quantifies these contaminants, providing information on pollution levels and their impact on the environment[184,185]. For instance, this approach has been used to monitor the presence of residues, such as antibiotics, hormones, and opioids, which may have adverse effects on human health and aquatic ecosystems, enabling the identification of regions where pollution is most severe and providing evidence to guide environmental policies and remediation efforts[184,185].
Finally, WBE has been used to identify emerging infectious threats, such as viruses with zoonotic potential, which can be detected in wastewaters before they trigger widespread outbreaks. Through the analysis of genomic traces of these unknown or newly circulating pathogens in sewage systems, public health authorities can take proactive actions to mitigate risks[9,54]. For instance, zoonotic diseases, such as avian influenza or coronaviruses, can be transmitted to human populations without being immediately undetected[186]. WBE provides a scalable, early detection method for identifying these events, particularly in regions with close interactions between humans and animals[147]. Integrating WBE with other complementary surveillance methods (e.g., Aerobiology and clinical diagnostics) enables more effective monitoring of the evolution and transmission of these emerging pathogens[9].
BUILDING RESILIENCE: ADDRESSING CHALLENGES AND CHARTING THE FUTURE OF WASTEWATER SURVEILLANCE
Current challenges
One of the primary challenges of WBE is associated with the technical complexities involved in detecting and analyzing pathogens or contaminants in this type of sample. Wastewater is a highly heterogeneous medium containing several chemical, biological, and environmental components that can interfere with the adopted detection methods[14]. The degradation of genetic material, specifically viral RNA, due to environmental factors (e.g., temperature, pH, and microbial activity), coupled with the low concentrations of target analytes and the presence of inhibitory compounds, can compromise the accuracy of results[14,187]. Moreover, reliable pathogen detection is deeply dependent on robust molecular tools, such as RT-qPCR and NGS analysis, which demand specialized equipment, reagents, and skilled personnel[89].
Secondly, while significant advances have been made to ensure standardization during sample collection, processing, and analysis across different regions, these processes remain challenging. Variations in sewage infrastructure, population density, and sampling strategies lead to inconsistencies that make it difficult to compare results on a global scale. Addressing these issues requires the establishment of universal protocols and quality control measures to ensure reliable and reproducible data[188,189].
The implementation of WBE at a global scale is also associated with significant logistical barriers. On one hand, wastewater sampling often requires access to centralized sewage systems, which may not be available in all regions, particularly in rural or low-income countries. Informal settlements and areas with fragmented sanitation infrastructure lack the ability to support consistent sampling, leading to surveillance coverage gaps[190]. On the other hand, transporting, storing, and analyzing samples requires careful coordination since delays in sample processing result in the degradation of genetic material, which reduces detection sensitivity[191]. The development of reliable sampling networks, which ensure timely sample collection and analysis, constitutes a significant logistical hurdle, particularly in regions with limited public health infrastructure[192].
While WBE is often considered a cost-effective approach compared to large-scale individual testing, its widespread adoption requires significant financial investment in infrastructure, technology, and human resources[193]. Nevertheless, establishing WBE protocols involves the acquisition of equipment, settlement of adequate laboratory facilities, and several ongoing operational expenses, such as reagents, sample transport, and data analysis. For LMICs, these financial demands can be prohibitive, limiting their ability to implement WBE programs on a large scale[133]. The long-term sustainability of WBE depends on continued financial support from governments, public health organizations, and international entities[39]. The lack of this sustained investment leads to the abandonment of WBE programs, particularly during non-pandemic situations, potentially compromising their effectiveness in future public health crises [Table 4].
Challenges and mitigation strategies for WBE implementation
| Challenge | Description | Proposed solution | Stakeholders involved |
| Lack of infrastructure | Absence of centralized sewage systems in some regions | Use of localized sampling or on-site treatment plant access | Local governments, NGOs |
| Limited laboratory capacity | Shortage of trained personnel or equipment for RT-qPCR/NGS | Investment in training and mobile labs | Ministries of Health, academia |
| Sample degradation during transport | RNA/DNA decay due to poor storage and long delays | Cold-chain logistics; field-based pre-treatment | Donor agencies, logistics partners |
| Inconsistent data reporting | Lack of harmonized standards across regions | Develop global WBE guidelines and metadata templates | WHO, CDC, surveillance consortia |
| Limited funding | High setup and maintenance costs for sustainable monitoring | Long-term donor support; integration into health budgets | Governments, WHO, international donors |
Global collaboration
The success of WBE as a global surveillance tool deeply depends on the collaboration between countries, institutions, and disciplines. To maximize its effectiveness, robust systems for data sharing, establishing international standards, and adopting interdisciplinary approaches are required. These elements will undoubtedly harmonize the methods used, improve results’ comparability, and ensure timely and coordinated responses to global public health challenges[194].
Effective WBE depends on collecting, analyzing, and disseminating pathogen data at local, national, and international levels. However, the variability in sampling methods, processing techniques, and reporting practices across different regions limits the comparison and interpretation of these worldwide findings. Establishing standards and guidelines for sample collection, pathogen detection, and data analysis applicable at a global scale ensures consistency, reliability, and reproducibility of results across borders. These efforts should be driven by organizations such as the WHO and constitute a critical point for aligning public health strategies on a global scale[8,9].
Moreover, data sharing platforms promote collaboration and transparency[2]. Open-access repositories and networks allow researchers and policymakers to share wastewater data in near real time, enhancing the capacity to track pathogen evolution and dissemination and improving the early detection of emerging pathogens. For instance, by November 2022, 14 million SARS-CoV-2 genomic sequences were available in public databases, facilitating near real-time viral evolution study, diagnostic assay assessment, and vaccine efficacy improvement[195].
The complexity of wastewater surveillance also requires an interdisciplinary approach that brings together experts from diverse fields of knowledge, such as microbiology, environmental science, bioinformatics, public health, and engineering. The collaboration across these fields allows for the development of innovative technologies, such as advanced sequencing methods and predictive modeling tools, to improve pathogen detection and analysis[90].
Initiatives such as the MetaSUB Consortium illustrate the power of global and interdisciplinary collaboration. This consortium combines expertise from multiple scientific fields to develop a comprehensive approach to urban microbial surveillance that integrates data from wastewater, air, and surface sampling. This holistic strategy enhances the understanding of microbial ecosystems and provides insights into pathogen transmission routes and AMR in urban environments. This international network has created a collaborative model for global disease surveillance, leveraging shared resources and technologies, and demonstrated how cross-border cooperation could overcome the challenges of fragmented health infrastructure and data silos, contributing to pandemic preparedness and improved public health outcomes[20].
Future opportunities
For WBE to be integrated into public health frameworks, significant policy changes should occur at national and international levels [Figure 5]. Governments and health organizations should prioritize wastewater monitoring as a core component of disease surveillance systems. This includes creating regulatory frameworks that mandate wastewater testing during health emergencies and establishing protocols for routine surveillance to monitor known and emerging threats[104].
Figure 5. Strategic roadmap for integrating wastewater-based epidemiology (WBE) into global health surveillance.
Sustained funding to support WBE infrastructure development and maintenance should be applied to improve laboratory capacity, purchase specialized equipment, and train personnel in wastewater sampling and analysis techniques[196-198]. International organizations, such as the WHO and global funding entities, should provide financial assistance to regions with limited resources, while partnerships between governments, non-governmental organizations, and private sectors should further ensure the longevity of WBE programs[199].
In terms of infrastructure development, expanding sewage networks and ensuring equitable access to sanitation systems is critical, and investments should be directed to modernize wastewater treatment plants and implement automated sampling technologies that will enable more reliable and scalable data collection[200,201]. Moreover, integrating WBE into existing public health systems, such as national disease control programs, will facilitate data sharing and improve coordination among stakeholders.
WBE holds great potential in high-income nations[108,132]. However, its scalability for LMICs presents both challenges and opportunities. Many of these countries face barriers such as fragmented sewage systems, limited laboratory infrastructure, and financial constraints[132,202]. Addressing these challenges requires targeted strategies to make WBE more accessible, adaptable, and sustainable.
First, simplifying sampling and testing protocols ensures that wastewater surveillance can be easily implemented without requiring advanced technologies or highly specialized expertise[203-205]. Portable, low-cost testing kits that provide rapid and reliable results could significantly enhance WBE accessibility in resource-limited settings[206,207]. Collaborative efforts to develop and distribute such tools would bridge the technology gap, enabling widespread adoption.
Second, it is important to build local capacity through training and education programs focused on developing the skills of public health professionals, engineers, and laboratory technicians, and empowering local communities to conduct wastewater monitoring independently. International partnerships and knowledge-sharing initiatives can further accelerate capacity building, ensuring that LMICs can establish and maintain effective surveillance networks[208,209].
Finally, integrating WBE into global health initiatives will scale up its use in LMICs. The incorporation of wastewater surveillance into frameworks such as the Global Health Security Agenda (GHSA) or WHO-coordinated disease surveillance systems will allow LMICs to benefit from financial, technical, and operational support. These partnerships ensure that WBE will be deployed during pandemics and will become a routine part of long-term public health strategies.
CONCLUSION
WBE has been proven to be a groundbreaking approach for tracking infectious diseases, detecting AMR, and identifying environmental pollutants. The analysis of pathogens shed in human waste provides early warning systems for disease outbreaks, often detecting infections before the first clinical cases are reported. This capability positions WBE as a critical tool for modern public health systems, enabling authorities to promptly respond to emerging health threats.
The integration of WBE with Aerobiology enhances the overall effectiveness of surveillance efforts, providing additional insights into respiratory pathogen circulation, validating findings from wastewater, and offering a more comprehensive view of disease transmission in communities. When combined, these tools deliver a multi-layered surveillance system that improves the accuracy and reliability of early detection strategies.
Global collaborations, such as the MetaSUB Consortium, emphasize the importance of interdisciplinary and international partnerships for environmental surveillance. By combining data from wastewater, air, and surface sampling, MetaSUB has demonstrated how global networks can advance the understanding of pathogen dissemination in urban settings. The consortium’s contributions during the COVID-19 pandemic underscored the role of environmental monitoring in tracking infections, identifying hotspots, and guiding public health interventions.
Despite its success, WBE also presents its limitations, particularly in terms of infrastructure, funding, and standardization. Addressing these constraints will ensure its scalability and effectiveness worldwide, especially in regions with limited resources. Investing in improved sewage systems, laboratory capacity, and workforce training will allow WBE to become a routine component of public health strategies, offering long-term disease monitoring and prevention benefits.
To fully harness the potential of WBE, policymakers and public health officials must prioritize its integration into national and global health surveillance systems. By investing in infrastructure, standardizing methodologies, and supporting interdisciplinary collaboration, WBE has the potential to become a cornerstone of early warning strategies, enhancing outbreak preparedness, reducing response times, and protecting public health globally.
As concluding remarks, WBE, particularly when combined with other tools, such as Aerobiology and highly touched surface analysis, and strengthened by international initiatives (e.g., Meta SUB) or global organizations (e.g., WHO), represents a scalable and proactive solution for global health surveillance. By enabling early detection of emerging threats and providing actionable data, WBE holds the potential to enhance pandemic preparedness, protect public health, and strengthen global resilience against future disease outbreaks.
DECLARATIONS
Authors’ contributions
Conceived of the presented manuscript and was responsible for methodology, formal analysis, investigation, resources, writing - original draft preparation, writing, reviewing, editing, and visualization: Oliveira, M.
Writing, reviewing, and editing: Prithiviraj, B.; Osuolale, O. O.; Ugalde, J. A.; Bhattacharyya, M.; Dinis-Oliveira, R. J.; Madureira-Carvalho, Á.; Dias da Silva, D.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
This study received support and help from FCT/MCTES in the scope of the projects UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences - UCIBIO; the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy - i4HB; and LAQV (LA/P/0008/2020 DOI 10.54499/LA/P/0008/2020, UIDP/50006/2020 DOI 10.54499/UIDP/50006/2020 and UIDB/50006/2020 DOI 10.54499/UIDB/50006/2020).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Ramos, P. I. P.; Marcilio, I.; Bento, A. I.; et al; ÆSOP Collaborating Teams. Combining digital and molecular approaches using health and alternate data sources in a next-generation surveillance system for anticipating outbreaks of pandemic potential. JMIR. Public. Health. Surveill.2024, 10, e47673.
2. Grieve, R.; Yang, Y.; Abbott, S.; et al. The importance of investing in data, models, experiments, team science, and public trust to help policymakers prepare for the next pandemic. PLOS. Glob. Public. Health. 2023, 3, e0002601.
3. Vianello, C.; Strozzi, F.; Mocellin, P.; et al. A perspective on early detection systems models for COVID-19 spreading. Biochem. Biophys. Res. Commun. 2021, 538, 244-52.
4. MacAulay, S.; Ellison, A. R.; Kille, P.; Cable, J. Moving towards improved surveillance and earlier diagnosis of aquatic pathogens: from traditional methods to emerging technologies. Rev. Aquac. 2022, 14, 1813-29.
5. Syal, K. Guidelines on newly identified limitations of diagnostic tools for COVID-19 and consequences. J. Med. Virol. 2021, 93, 1837-42.
6. Hierink, F.; Okiro, E. A.; Flahault, A.; Ray, N. The winding road to health: a systematic scoping review on the effect of geographical accessibility to health care on infectious diseases in low- and middle-income countries. PLoS. One. 2021, 16, e0244921.
7. Pasquale, S.; Gregorio, G. L.; Caterina, A.; et al. COVID-19 in low- and middle-income countries (LMICs): a narrative review from prevention to vaccination strategy. Vaccines 2021, 9, 1477.
8. Huizer, M.; Ter Laak, T. L.; de Voogt, P.; van Wezel, A. P. Wastewater-based epidemiology for illicit drugs: a critical review on global data. Water. Res. 2021, 207, 117789.
9. Sims, N.; Kasprzyk-Hordern, B. Future perspectives of wastewater-based epidemiology: monitoring infectious disease spread and resistance to the community level. Environ. Int. 2020, 139, 105689.
10. Parkins, M. D.; Lee, B. E.; Acosta, N.; et al. Wastewater-based surveillance as a tool for public health action: SARS-CoV-2 and beyond. Clin. Microbiol. Rev. 2024, 37, e0010322.
11. Robins, K.; Leonard, A. F. C.; Farkas, K.; et al. Research needs for optimising wastewater-based epidemiology monitoring for public health protection. J. Water. Health. 2022, 20, 1284-313.
12. Farkas, K.; Kevill, J. L.; Williams, R. C.; et al. Comparative assessment of Nanotrap and polyethylene glycol-based virus concentration in wastewater samples. FEMS. Microbes. 2024, 5, xtae007.
13. de Lourdes Aguiar-Oliveira, M. L.; Campos, A.; Matos, A. R.; et al. Wastewater-based epidemiology (WBE) and viral detection in polluted surface water: a valuable tool for COVID-19 surveillance - a brief review. Int. J. Environ. Res. Public. Health. 2020, 17, 9251.
14. Kumblathan, T.; Liu, Y.; Uppal, G. K.; Hrudey, S. E.; Li, X. F. Wastewater-based epidemiology for community monitoring of SARS-CoV-2: progress and challenges. ACS. Environ. Au. 2021, 1, 18-31.
15. Mousazadeh, M.; Ashoori, R.; Paital, B.; et al. Wastewater based epidemiology perspective as a faster protocol for detecting coronavirus RNA in human populations: a review with specific reference to SARS-CoV-2 virus. Pathogens 2021, 10, 1008.
16. Picó, Y.; Barceló, D. Mass spectrometry in wastewater-based epidemiology for the determination of small and large molecules as biomarkers of exposure: toward a global view of environment and human health under the COVID-19 outbreak. ACS. Omega. 2021, 6, 30865-72.
17. Gitter, A.; Oghuan, J.; Godbole, A. R.; et al. Not a waste: wastewater surveillance to enhance public health. Front. Chem. Eng. 2023, 4, 1112876.
18. Tharak, A.; Kopperi, H.; Hemalatha, M.; et al. Longitudinal and long-term wastewater surveillance for COVID-19: infection dynamics and zoning of urban community. Int. J. Environ. Res. Public. Health. 2022, 19, 2697.
19. Tignat-Perrier, R.; Dommergue, A.; Vogel, T. M.; Larose, C. Microbial ecology of the planetary boundary layer. Atmosphere 2020, 11, 1296.
20. Ryon, K. A.; Tierney, B. T.; Frolova, A.; et al. A history of the MetaSUB consortium: tracking urban microbes around the globe. iScience 2022, 25, 104993.
21. Miranda, M. N. S.; Pingarilho, M.; Pimentel, V.; et al. A tale of three recent pandemics: influenza, HIV and SARS-CoV-2. Front. Microbiol. 2022, 13, 889643.
23. Han, J. J.; Song, H. A.; Pierson, S. L.; Shen-Gunther, J.; Xia, Q. Emerging infectious diseases are virulent viruses - are we prepared? An overview. Microorganisms 2023, 11, 2618.
24. Roychoudhury, S.; Das, A.; Sengupta, P.; et al. Viral pandemics of the last four decades: pathophysiology, health impacts and perspectives. Int. J. Environ. Res. Public. Health. 2020, 17, 9411.
25. Wu, D. D.; Mitchell, J.; Lambert, J. H. Global systemic risk and resilience for novel coronavirus and COVID-19. Risk. Anal. 2021, 41, 701-4.
27. Bassetti, M.; Vena, A.; Giacobbe, D. R. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur. J. Clin. Invest. 2020, 50, e13209.
28. Kupferschmidt, K.; Cohen, J. Will novel virus go pandemic or be contained? Science 2020, 367, 610-1.
30. National Academies of Sciences, Engineering, and Medicine and National Academy of Medicine. Countering the pandemic threat through global coordination on vaccines: the influenza imperative. 2022. https://nap.nationalacademies.org/catalog/26284/countering-the-pandemic-threat-through-global-coordination-on-vaccines-the. (accessed 8 May 2025).
31. Nobles, M.; Lall, R.; Mathes, R. W.; Neill, D. B. Presyndromic surveillance for improved detection of emerging public health threats. Sci. Adv. 2022, 8, eabm4920.
32. Moghadas, S. M.; Fitzpatrick, M. C.; Sah, P.; et al. The implications of silent transmission for the control of COVID-19 outbreaks. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 17513-5.
33. Chala, B.; Hamde, F. Emerging and re-emerging vector-borne infectious diseases and the challenges for control: a review. Front. Public. Health. 2021, 9, 715759.
34. Downie, D. L.; Rao, P.; David-Ferdon, C.; et al. Surveillance for emerging and reemerging pathogens using pathogen agnostic metagenomic sequencing in the United States: a critical role for federal government agencies. Health. Secur. 2024, 22, 85-92.
35. Donia, A.; Hassan, S. U.; Zhang, X.; Al-Madboly, L.; Bokhari, H. COVID-19 crisis creates opportunity towards global monitoring & surveillance. Pathogens 2021, 10, 256.
36. Mackuľak, T.; Gál, M.; Špalková, V.; et al. Wastewater-based epidemiology as an early warning system for the spreading of SARS-CoV-2 and its mutations in the population. Int. J. Environ. Res. Public. Health. 2021, 18, 5629.
37. Levy, J. I.; Andersen, K. G.; Knight, R.; Karthikeyan, S. Wastewater surveillance for public health. Science 2023, 379, 26-7.
38. Gupta, S.; Gupta, T.; Gupta, N. Global respiratory virus surveillance: strengths, gaps, and way forward. Int. J. Infect. Dis. 2022, 121, 184-9.
39. Bergeri, I.; Lewis, H. C.; Subissi, L.; et al. Early epidemiological investigations: World Health Organization UNITY protocols provide a standardized and timely international investigation framework during the COVID-19 pandemic. Influenza. Other. Respir. Viruses. 2022, 16, 7-13.
40. Kasprzyk-Hordern, B.; Béen, F.; Bijlsma, L.; et al. Wastewater-based epidemiology for the assessment of population exposure to chemicals: The need for integration with human biomonitoring for global One Health actions. J. Hazard. Mater. 2023, 450, 131009.
41. Wenham, C.; Davies, S. E. WHO runs the world - (not) girls: gender neglect during global health emergencies. Int. Fem. J. Polit. 2022, 24, 415-38.
42. Hahn, R. Z.; Augusto do Nascimento, C.; Linden, R. Evaluation of illicit drug consumption by wastewater analysis using polar organic chemical integrative sampler as a monitoring tool. Front. Chem. 2021, 9, 596875.
43. Tiwari, A.; Kurittu, P.; Al-Mustapha, A. I.; et al; WastPan Study Group. Wastewater surveillance of antibiotic-resistant bacterial pathogens: a systematic review. Front. Microbiol.2022, 13, 977106.
44. Izquierdo-Lara, R.; Elsinga, G.; Heijnen, L.; et al. Monitoring SARS-CoV-2 circulation and diversity through community wastewater sequencing, the Netherlands and Belgium. Emerg. Infect. Dis. 2021, 27, 1405-15.
45. Bar-Or, I.; Yaniv, K.; Shagan, M.; et al. Regressing SARS-CoV-2 sewage measurements onto COVID-19 burden in the population: a proof-of-concept for quantitative environmental surveillance. Front. Public. Health. 2021, 9, 561710.
46. Boeraș, I.; Curtean-Bănăduc, A.; Bănăduc, D.; Cioca, G. Anthropogenic sewage water circuit as vector for SARS-CoV-2 viral ARN transport and public health assessment, monitoring and forecasting - Sibiu Metropolitan Area (Transylvania/Romania) Study Case. Int. J. Environ. Res. Public. Health. 2022, 19, 11725.
47. McGowan, J.; Borucki, M.; Omairi, H.; et al. SARS-CoV-2 monitoring in wastewater reveals novel variants and biomarkers of infection. Viruses 2022, 14, 2032.
48. Henriques, T. B.; Cassini, S. T.; de Pinho Keller, R. Contribution of wastewater-based epidemiology to SARS-CoV-2 screening in Brazil and the United States. J. Water. Health. 2023, 21, 343-53.
49. Ash, K. T.; Li, Y.; Alamilla, I.; et al. SARS-CoV-2 raw wastewater surveillance from student residences on an urban university campus. Front. Microbiol. 2023, 14, 1101205.
50. Innes, G. K.; Schmitz, B. W.; Brierley, P. E.; et al. Wastewater-based epidemiology mitigates COVID-19 outbreaks at a food processing facility near the Mexico-U.S. Border-November 2020-March 2022. Viruses 2022, 14, 2684.
51. Lovell-Read, F. A.; Funk, S.; Obolski, U.; Donnelly, C. A.; Thompson, R. N. Interventions targeting non-symptomatic cases can be important to prevent local outbreaks: SARS-CoV-2 as a case study. J. R. Soc. Interface. 2021, 18, 20201014.
52. Nichols, J. D.; Bogich, T. L.; Howerton, E.; et al. Strategic testing approaches for targeted disease monitoring can be used to inform pandemic decision-making. PLoS. Biol. 2021, 19, e3001307.
53. Dos Santos, J. L. G.; Stein Messetti, P. A.; Adami, F.; et al. Collision of fundamental human rights and the right to health access during the novel coronavirus pandemic. Front. Public. Health. 2020, 8, 570243.
54. Zahedi, A.; Monis, P.; Deere, D.; Ryan, U. Wastewater-based epidemiology-surveillance and early detection of waterborne pathogens with a focus on SARS-CoV-2, Cryptosporidium and Giardia. Parasitol. Res. 2021, 120, 4167-88.
55. Carmo Dos Santos, M.; Cerqueira Silva, A. C.; Dos Reis Teixeira, C.; et al. Wastewater surveillance for viral pathogens: a tool for public health. Heliyon 2024, 10, e33873.
56. Coggon, J. Is public health just science? Values, politics and varied but collective practices to secure better health with justice. J. Public. Health. 2022, 44, i34-9.
57. Tomori, C.; Evans, D. P.; Ahmed, A.; Nair, A.; Meier, B. M. Where is the “Public” in American Public Health? Moving from individual responsibility to collective action. EClinicalMedicine 2022, 45, 101341.
58. Qiu, G.; Zhang, X.; deMello, A. J.; Yao, M.; Cao, J.; Wang, J. On-site airborne pathogen detection for infection risk mitigation. Chem. Soc. Rev. 2023, 52, 8531-79.
59. Comber, L.; Murchu, E. O.; Drummond, L.; et al. Airborne transmission of SARS-CoV-2 via aerosols. Rev. Med. Virol. 2021, 31, e2184.
60. Domínguez-Amarillo, S.; Fernández-Agüera, J.; Cesteros-García, S.; González-Lezcano, R. A. Bad air can also kill: residential indoor air quality and pollutant exposure risk during the COVID-19 crisis. Int. J. Environ. Res. Public. Health. 2020, 17, 7183.
61. Kadadou, D.; Tizani, L.; Wadi, V. S.; et al. Recent advances in the biosensors application for the detection of bacteria and viruses in wastewater. J. Environ. Chem. Eng. 2022, 10, 107070.
62. Mao, K.; Zhang, H.; Pan, Y.; Yang, Z. Biosensors for wastewater-based epidemiology for monitoring public health. Water. Res. 2021, 191, 116787.
63. Memon, R.; Niazi, J. H.; Qureshi, A. Biosensors for detection of airborne pathogenic fungal spores: a review. Nanoscale 2024, 16, 15419-45.
64. Rastmanesh, A.; Boruah, J. S.; Lee, M. S.; Park, S. On-site bioaerosol sampling and airborne microorganism detection technologies. Biosensors 2024, 14, 122.
65. Danko, D.; Bezdan, D.; Afshin, E. E.; et al; International MetaSUB Consortium. A global metagenomic map of urban microbiomes and antimicrobial resistance. Cell.2021, 184, 3376-93.e17.
66. Farkas, K.; Walker, D. I.; Adriaenssens, E. M.; et al. Viral indicators for tracking domestic wastewater contamination in the aquatic environment. Water. Res. 2020, 181, 115926.
67. Chng, K. R.; Li, C.; Bertrand, D.; et al; MetaSUB Consortium. Cartography of opportunistic pathogens and antibiotic resistance genes in a tertiary hospital environment. Nat. Med. 2020, 26, 941-51.
68. Zhang, N.; Su, B.; Chan, P. T.; Miao, T.; Wang, P.; Li, Y. Infection spread and high-resolution detection of close contact behaviors. Int. J. Environ. Res. Public. Health. 2020, 17, 1445.
69. Di Battista, A.; Nicolaides, C.; Georgiou, O. Modelling disease transmission from touchscreen user interfaces. R. Soc. Open. Sci. 2021, 8, 210625.
70. Contreras, D. A.; Colosi, E.; Bassignana, G.; Colizza, V.; Barrat, A. Impact of contact data resolution on the evaluation of interventions in mathematical models of infectious diseases. J. R. Soc. Interface. 2022, 19, 20220164.
71. Stewart, J. D.; Kremer, P.; Shakya, K. M.; Conway, M.; Saad, A. Outdoor atmospheric microbial diversity is associated with urban landscape structure and differs from indoor-transit systems as revealed by mobile monitoring and three-dimensional spatial analysis. Front. Ecol. Evol. 2021, 9, 620461.
72. Toepfer, M.; Herrmann, B.; Sansone, M.; Lilja, C.; Nolskog, P. Environmental contamination by chlamydia trachomatis RNA can cause false-positive test results in clinical samples. Sex. Transm. Dis. 2021, 48, e88-90.
73. Marcenac, P.; Park, G. W.; Duca, L. M.; et al. Detection of SARS-CoV-2 on surfaces in households of persons with COVID-19. Int. J. Environ. Res. Public. Health. 2021, 18, 8184.
74. Rose, L. J.; Houston, H.; Martinez-Smith, M.; et al. Factors influencing environmental sampling recovery of healthcare pathogens from non-porous surfaces with cellulose sponges. PLoS. One. 2022, 17, e0261588.
75. Bento de Carvalho, T.; Barbosa, J. B.; Teixeira, P. Assessing antimicrobial efficacy on plastics and other non-porous surfaces: a closer look at studies using the ISO 22196:2011 standard. Biology 2024, 13, 59.
76. Sterzenbach, T.; Neumann, V.; Trips, E.; Basche, S.; Hannig, C.; Kühne, M. T. Establishment of a protocol for viability qPCR in dental hard tissues. Microorganisms 2024, 12, 1400.
77. Freitas, B. L.; Leach, L.; Chaturvedi, V.; Chaturvedi, S. Reverse transcription-quantitative real-time PCR (RT-qPCR) assay for the rapid enumeration of live Candida auris cells from the health care environment. J. Clin. Microbiol. 2022, 60, e0077921.
78. Thilakarathna, S. H.; Stokowski, T.; Chui, L. An improved real-time viability PCR assay to detect salmonella in a culture-independent era. Int. J. Mol. Sci. 2022, 23, 14708.
79. Kolimenakis, A.; Heinz, S.; Wilson, M. L.; et al. The role of urbanisation in the spread of Aedes mosquitoes and the diseases they transmit - a systematic review. PLoS. Negl. Trop. Dis. 2021, 15, e0009631.
80. Hassell, J. M.; Muloi, D. M.; VanderWaal, K. L.; et al. Epidemiological connectivity between humans and animals across an urban landscape. Proc. Natl. Acad. Sci. U. S. A. 2023, 120, e2218860120.
81. Bhattacharya, C.; Tierney, B. T.; Ryon, K. A.; et al. Supervised machine learning enables geospatial microbial provenance. Genes 2022, 13, 1914.
82. Sojobi, A. O.; Zayed, T. Impact of sewer overflow on public health: a comprehensive scientometric analysis and systematic review. Environ. Res. 2022, 203, 111609.
83. Murakami, M.; Kitajima, M.; Endo, N.; Ahmed, W.; Gawlik, B. M. The growing need to establish a global wastewater surveillance consortium for future pandemic preparedness. J. Travel. Med. 2023, 30, taad035.
84. Gahlot, P.; Alley, K. D.; Arora, S.; Das, S.; Nag, A.; Tyagi, V. K. Wastewater surveillance could serve as a pandemic early warning system for COVID-19 and beyond. WIREs. Water. 2023, 10, e1650.
85. Corrin, T.; Rabeenthira, P.; Young, K. M.; et al. A scoping review of human pathogens detected in untreated human wastewater and sludge. J. Water. Health. 2024, 22, 436-49.
86. Augusto, M. R.; Claro, I. C. M.; Siqueira, A. K.; et al. Sampling strategies for wastewater surveillance: evaluating the variability of SARS-COV-2 RNA concentration in composite and grab samples. J. Environ. Chem. Eng. 2022, 10, 107478.
87. Chau, K. K.; Goodall, T.; Bowes, M.; et al. High-resolution characterization of short-term temporal variability in the taxonomic and resistome composition of wastewater influent. Microb. Genom. 2023, 9, mgen000983.
88. Stokdyk, J. P.; Firnstahl, A. D.; Walsh, J. F.; et al. Viral, bacterial, and protozoan pathogens and fecal markers in wells supplying groundwater to public water systems in Minnesota, USA. Water. Res. 2020, 178, 115814.
89. Garner, E.; Davis, B. C.; Milligan, E.; et al. Next generation sequencing approaches to evaluate water and wastewater quality. Water. Res. 2021, 194, 116907.
90. Singh, R.; Ryu, J.; Hyoung, L. W.; Kang, J. H.; Park, S.; Kim, K. Wastewater-borne viruses and bacteria, surveillance and biosensors at the interface of academia and field deployment. Crit. Rev. Biotechnol. 2025, 45, 413-33.
91. Child, H. T.; O’Neill, P. A.; Moore, K.; et al. Optimised protocol for monitoring SARS-CoV-2 in wastewater using reverse complement PCR-based whole-genome sequencing. PLoS. One. 2023, 18, e0284211.
92. Gonzalez, R.; Curtis, K.; Bivins, A.; et al. COVID-19 surveillance in Southeastern Virginia using wastewater-based epidemiology. Water. Res. 2020, 186, 116296.
93. Jahne, M. A.; Brinkman, N. E.; Keely, S. P.; Zimmerman, B. D.; Wheaton, E. A.; Garland, J. L. Droplet digital PCR quantification of norovirus and adenovirus in decentralized wastewater and graywater collections: implications for onsite reuse. Water. Res. 2020, 169, 115213.
94. McCall, C.; Wu, H.; Miyani, B.; Xagoraraki, I. Identification of multiple potential viral diseases in a large urban center using wastewater surveillance. Water. Res. 2020, 184, 116160.
95. Tao, J.; Liu, W.; Ding, W.; et al. A multiplex PCR assay with a common primer for the detection of eleven foodborne pathogens. J. Food. Sci. 2020, 85, 744-54.
96. Falzone, L.; Musso, N.; Gattuso, G.; et al. Sensitivity assessment of droplet digital PCR for SARS-CoV-2 detection. Int. J. Mol. Med. 2020, 46, 957-64.
97. dMIQE Group; Huggett, J. F. The Digital MIQE Guidelines update: minimum information for publication of quantitative digital PCR experiments for 2020. Clin. Chem. 2020, 66, 1012-29.
98. Mirabile, A.; Sangiorgio, G.; Bonacci, P. G.; et al. Advancing pathogen identification: the role of digital PCR in enhancing diagnostic power in different settings. Diagnostics 2024, 14, 1598.
99. Safford, H. R.; Shapiro, K.; Bischel, H. N. Opinion: Wastewater analysis can be a powerful public health tool-if it’s done sensibly. Proc. Natl. Acad. Sci. U. S. A. 2022, 119, e2119600119.
100. Randazzo, W.; Cuevas-Ferrando, E.; Sanjuán, R.; Domingo-Calap, P.; Sánchez, G. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. Int. J. Hyg. Environ. Health. 2020, 230, 113621.
101. Peccia, J.; Zulli, A.; Brackney, D. E.; et al. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020, 38, 1164-7.
102. Martin, J.; Klapsa, D.; Wilton, T.; et al. Tracking SARS-CoV-2 in sewage: evidence of changes in virus variant predominance during COVID-19 pandemic. Viruses 2020, 12, 1144.
103. Keshaviah, A.; Hu, X. C.; Henry, M. Developing a flexible national wastewater surveillance system for COVID-19 and beyond. Environ. Health. Perspect. 2021, 129, 45002.
104. Raza, S.; Shin, H.; Hur, H. G.; Unno, T. Higher abundance of core antimicrobial resistant genes in effluent from wastewater treatment plants. Water. Res. 2022, 208, 117882.
105. Conco, T.; Kumari, S.; Awolusi, O. O.; et al. Profiling of emerging pathogens, antibiotic resistance genes and mobile genetic elements in different biological wastewater treatment plants. J. Environ. Chem. Eng. 2022, 10, 107596.
106. Chavarria-Miró, G.; Anfruns-Estrada, E.; Martínez-Velázquez, A.; et al. Time evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in wastewater during the first pandemic wave of COVID-19 in the metropolitan area of Barcelona, Spain. Appl. Environ. Microbiol. 2021, 87, e02750-20.
107. Alhama, J.; Maestre, J. P.; Martín, M. Á.; Michán, C. Monitoring COVID-19 through SARS-CoV-2 quantification in wastewater: progress, challenges and prospects. Microb. Biotechnol. 2022, 15, 1719-28.
108. Gonçalves, J.; Torres-Franco, A.; Rodriguéz, E.; et al. Centralized and decentralized wastewater-based epidemiology to infer COVID-19 transmission - a brief review. One. Health. 2022, 15, 100405.
109. Fanelli, S.; Salvatore, F. P.; De Pascale, G.; Faccilongo, N. Insights for the future of health system partnerships in low- and middle-income countries: a systematic literature review. BMC. Health. Serv. Res. 2020, 20, 571.
110. Ramuta, M. D.; Newman, C. M.; Brakefield, S. F.; et al. SARS-CoV-2 and other respiratory pathogens are detected in continuous air samples from congregate settings. Nat. Commun. 2022, 13, 4717.
111. Anand, U.; Adelodun, B.; Pivato, A.; et al. A review of the presence of SARS-CoV-2 RNA in wastewater and airborne particulates and its use for virus spreading surveillance. Environ. Res. 2021, 196, 110929.
112. Xagoraraki, I.; O’Brien, E. Wastewater-based epidemiology for early detection of viral outbreaks. In: O’Bannon DJ, editor. Women in water quality. Cham: Springer International Publishing; 2020. pp. 75-97.
113. Ahmed, W.; Tscharke, B.; Bertsch, P. M.; et al. SARS-CoV-2 RNA monitoring in wastewater as a potential early warning system for COVID-19 transmission in the community: a temporal case study. Sci. Total. Environ. 2021, 761, 144216.
114. Nwaubani, D. A.; Baral, R.; Solomon, T.; Idris, O.; Sherchan, S. P. Wastewater surveillance of Candida auris in Baltimore. Int. J. Hyg. Environ. Health. 2025, 263, 114486.
115. Markt, R.; Stillebacher, F.; Nägele, F.; et al. Expanding the pathogen panel in wastewater epidemiology to influenza and norovirus. Viruses 2023, 15, 263.
116. Jeamsripong, S.; Thaotumpitak, V.; Anuntawirun, S.; Roongrojmongkhon, N.; Atwill, E. R.; Hinthong, W. Molecular epidemiology of antimicrobial resistance and virulence profiles of Escherichia coli, Salmonella spp., and Vibrio spp. isolated from coastal seawater for aquaculture. Antibiotics 2022, 11, 1688.
117. Djordjevic, S. P.; Jarocki, V. M.; Seemann, T.; et al. Genomic surveillance for antimicrobial resistance - a One Health perspective. Nat. Rev. Genet. 2024, 25, 142-57.
118. Raju, N. P.; Ansari, A.; Patil, G.; et al. Antibiotic resistance dissemination and mapping in the environment through surveillance of wastewater. J. Basic. Microbiol. 2025, 65, e2400330.
119. Ligda, P.; Claerebout, E.; Kostopoulou, D.; et al. Cryptosporidium and Giardia in surface water and drinking water: animal sources and towards the use of a machine-learning approach as a tool for predicting contamination. Environ. Pollut. 2020, 264, 114766.
120. Ligda, P.; Mittas, N.; Kyzas, G. Z.; Claerebout, E.; Sotiraki, S. Machine learning and explainable artificial intelligence for the prevention of waterborne cryptosporidiosis and giardiosis. Water. Res. 2024, 262, 122110.
121. Farkas, K.; Williams, R. C.; Hillary, L. S.; et al. Harnessing the power of next-generation sequencing in wastewater-based epidemiology and global disease surveillance. Food. Environ. Virol. 2024, 17, 5.
122. Pronyk, P. M.; de Alwis, R.; Rockett, R.; et al. Advancing pathogen genomics in resource-limited settings. Cell. Genom. 2023, 3, 100443.
123. Diao, Z.; Zhang, Y.; Chen, Y.; et al. Assessing the quality of metagenomic next-generation sequencing for pathogen detection in lower respiratory infections. Clin. Chem. 2023, 69, 1038-49.
124. Newman, H.; Hardie, D. HIV-1 viral load testing in resource-limited settings: challenges and solutions for specimen integrity. Rev. Med. Virol. 2021, 31, e2165.
125. Nijhawan, A.; Howard, G. Associations between climate variables and water quality in low- and middle-income countries: a scoping review. Water. Res. 2022, 210, 117996.
126. Curtis, A. N.; Tiemann, J. S.; Douglass, S. A.; Davis, M. A.; Larson, E. R.; Yang, J. High stream flows dilute environmental DNA (eDNA) concentrations and reduce detectability. Divers. Distrib. 2021, 27, 1918-31.
127. Guo, Y.; Li, J.; O’Brien, J.; Sivakumar, M.; Jiang, G. Back-estimation of norovirus infections through wastewater-based epidemiology: a systematic review and parameter sensitivity. Water. Res. 2022, 219, 118610.
128. Vásquez-Dean, J.; Maza, F.; Morel, I.; Pulgar, R.; González, M. Microbial communities from arid environments on a global scale. A systematic review. Biol. Res. 2020, 53, 29.
129. Chapman, W. A. L.; Finnegan, N. J. The signature of climate in fluvial suspended sediment records. JGR. Earth. Surface. 2024, 129, e2023JF007429.
130. Fierer, N.; Holland-Moritz, H.; Alexiev, A.; et al. A metagenomic investigation of spatial and temporal changes in sewage microbiomes across a University campus. mSystems 2022, 7, e0065122.
131. Nguyen, K. H.; Smith, S.; Roundtree, A.; et al. Fecal indicators and antibiotic resistance genes exhibit diurnal trends in the Chattahoochee River: implications for water quality monitoring. Front. Microbiol. 2022, 13, 1029176.
132. Adhikari, S.; Halden, R. U. Opportunities and limits of wastewater-based epidemiology for tracking global health and attainment of UN sustainable development goals. Environ. Int. 2022, 163, 107217.
133. Annan, J.; Henderson, R.; Gray, M.; Clark, R. G.; Sarin, C.; Black, K. A review of wastewater-based epidemiology for the SARS-CoV-2 virus in rural, remote, and resource-constrained settings internationally: insights for implementation, research, and policy for first nations in Canada. Int. J. Environ. Res. Public. Health. 2024, 21, 1429.
134. Hamilton, K. A.; Wade, M. J.; Barnes, K. G.; Street, R. A.; Paterson, S. Wastewater-based epidemiology as a public health resource in low- and middle-income settings. Environ. Pollut. 2024, 351, 124045.
135. Schmiege, D.; Haselhoff, T.; Thomas, A.; Kraiselburd, I.; Meyer, F.; Moebus, S. Small-scale wastewater-based epidemiology (WBE) for infectious diseases and antibiotic resistance: a scoping review. Int. J. Hyg. Environ. Health. 2024, 259, 114379.
136. Liu, D.; Clemente, L.; Poirier, C.; et al. Real-time forecasting of the COVID-19 outbreak in chinese provinces: machine learning approach using novel digital data and estimates from mechanistic models. J. Med. Internet. Res. 2020, 22, e20285.
137. Hu, B.; Tao, Y.; Shao, Z.; et al. A comparison of blood pathogen detection among droplet digital PCR, metagenomic next-generation sequencing, and blood culture in critically Ill patients with suspected bloodstream infections. Front. Microbiol. 2021, 12, 641202.
138. Mao, Y.; Shisler, J. L.; Nguyen, T. H. Enhanced detection for antibiotic resistance genes in wastewater samples using a CRISPR-enriched metagenomic method. Water. Res. 2025, 274, 123056.
139. Ardabili, S.; Mosavi, A.; Ghamisi, P.; et al. COVID-19 outbreak prediction with machine learning. Algorithms 2020, 13, 249.
140. Alfred, R.; Obit, J. H. The roles of machine learning methods in limiting the spread of deadly diseases: a systematic review. Heliyon 2021, 7, e07371.
141. Baskar, G.; Nashath Omer, S.; Saravanan, P.; et al. Status and future trends in wastewater management strategies using artificial intelligence and machine learning techniques. Chemosphere 2024, 362, 142477.
142. Gokul, K.; Gokul, V.; Kaviyarasan, P.; Uma, J. Intelligent wastewater management system with cloud-based IOT and real-time control. In 2024 10th International Conference on Advanced Computing and Communication Systems (ICACCS), Coimbatore, India. Oct 23, 2024. IEEE; 2024. pp. 498-504.
143. Salem, R. M. M.; Saraya, M. S.; Ali-Eldin, A. M. T. An industrial cloud-based IoT system for real-time monitoring and controlling of wastewater. IEEE. Access. 2022, 10, 6528-40.
144. Adebayo, A. I.; Olubanjo, K. T.; Fadeke, A. M.; et al. From static sampling to dynamic insights: the future of water quality monitoring with sensors, IoT, and drones. Sci. World. J. 2025, 20, 454-66. https://www.researchgate.net/publication/390795142_From_Static_Sampling_to_Dynamic_Insights_The_Future_of_Water_Quality_Monitoring_with_Sensors_IoT_and_Drones_FROM_STATIC_SAMPLING_TO_DYNAMIC_INSIGHTS_THE_FUTURE_OF_WATER_QUALITY_MONITORING_WITH_SENSORS. (accessed 8 May 2025)
145. Meng, Y.; Zhang, Y.; Wang, S.; et al. Lessons learned in the development of a web-based surveillance reporting system and dashboard to monitor acute febrile illnesses in Guangdong and Yunnan Provinces, China, 2017-2019. Health. Secur. 2020, 18, S14-22.
146. Focosi, D.; Spezia, P. G.; Maggi, F. Online dashboards for SARS-CoV-2 wastewater-based epidemiology. Future. Microbiol. 2024, 19, 761-9.
147. Prado, T.; Rey-Benito, G.; Miagostovich, M. P.; et al. Wastewater-based epidemiology for preventing outbreaks and epidemics in Latin America - lessons from the past and a look to the future. Sci. Total. Environ. 2023, 865, 161210.
148. Carducci, A.; Federigi, I.; Lauretani, G.; et al. Critical needs for integrated surveillance: wastewater-based and clinical epidemiology in evolving scenarios with lessons learned from SARS-CoV-2. Food. Environ. Virol. 2024, 16, 38-49.
149. O’Keeffe, J. Wastewater-based epidemiology: current uses and future opportunities as a public health surveillance tool. Environ. Health. Rev. 2021, 64, 44-52.
150. Gagliano, E.; Biondi, D.; Roccaro, P. Wastewater-based epidemiology approach: the learning lessons from COVID-19 pandemic and the development of novel guidelines for future pandemics. Chemosphere 2023, 313, 137361.
151. Gonçalves, J. The role of smart technologies in wastewater-based epidemiology. J. Environ. Expo. Assess. 2023, 2, 18.
152. Nagelkerke, E.; Hetebrij, W. A.; Koelewijn, J. M.; et al. PCR standard curve quantification in an extensive wastewater surveillance program: results from the Dutch SARS-CoV-2 wastewater surveillance. Front. Public. Health. 2023, 11, 1141494.
153. Rusková, M.; Bučková, M.; Achs, A.; et al. Useful molecular tools for facing next pandemic events: effective sample preparation and improved RT-PCR for highly sensitive detection of SARS-CoV-2 in wastewater environment. Int. J. Hyg. Environ. Health. 2022, 245, 114017.
154. Ding, J.; Xu, X.; Deng, Y.; Zheng, X.; Zhang, T. Comparison of RT-ddPCR and RT-qPCR platforms for SARS-CoV-2 detection: implications for future outbreaks of infectious diseases. Environ. Int. 2024, 183, 108438.
155. de Abreu, V. A. C.; Perdigão, J.; Almeida, S. Metagenomic approaches to analyze antimicrobial resistance: an overview. Front. Genet. 2020, 11, 575592.
156. Das, B.; Emon, M. I.; Moumi, N. A.; et al. HT-ARGfinder: a comprehensive pipeline for identifying horizontally transferred antibiotic resistance genes and directionality in metagenomic sequencing data. Front. Environ. Sci. 2022, 10, 901917.
157. Zhao, H.; Yang, M.; Fan, X.; et al. A Metagenomic investigation of potential health risks and element cycling functions of bacteria and viruses in wastewater treatment plants. Viruses 2024, 16, 535.
158. Parker, K.; Wood, H.; Russell, J. A.; et al. Development and optimization of an unbiased, metagenomics-based pathogen detection workflow for infectious disease and biosurveillance applications. Trop. Med. Infect. Dis. 2023, 8, 121.
159. Singh, N. K.; Yadav, M.; Singh, V.; et al. Artificial intelligence and machine learning-based monitoring and design of biological wastewater treatment systems. Bioresour. Technol. 2023, 369, 128486.
160. Bragazzi, N. L.; Dai, H.; Damiani, G.; Behzadifar, M.; Martini, M.; Wu, J. How big data and artificial intelligence can help better manage the COVID-19 pandemic. Int. J. Environ. Res. Public. Health. 2020, 17, 3176.
161. Wang, Y.; Cheng, Y.; Liu, H.; et al. A review on applications of artificial intelligence in wastewater treatment. Sustainability 2023, 15, 13557.
162. Phan, T.; Brozak, S.; Pell, B.; et al. Making waves: integrating wastewater surveillance with dynamic modeling to track and predict viral outbreaks. Water. Res. 2023, 243, 120372.
163. Shausan, A.; Nazarathy, Y.; Dyda, A. Emerging data inputs for infectious diseases surveillance and decision making. Front. Digit. Health. 2023, 5, 1131731.
164. Badidi, E. Edge AI for early detection of chronic diseases and the spread of infectious diseases: opportunities, challenges, and future directions. Future. Internet. 2023, 15, 370.
165. Liu, Y.; Ramin, P.; Flores-Alsina, X.; Gernaey, K. V. Transforming data into actionable knowledge for fault detection, diagnosis and prognosis in urban wastewater systems with AI techniques: a mini-review. Process. Saf. Environ. Prot. 2023, 172, 501-12.
166. Ai, Y.; He, F.; Lancaster, E.; Lee, J. Application of machine learning for multi-community COVID-19 outbreak predictions with wastewater surveillance. PLoS. One. 2022, 17, e0277154.
167. Foroughi, M.; Arzehgar, A.; Seyedhasani, S. N.; Nadali, A.; Zoroufchi, B. K. Application of machine learning for antibiotic resistance in water and wastewater: a systematic review. Chemosphere 2024, 358, 142223.
168. Chaibun, T.; Puenpa, J.; Ngamdee, T.; et al. Rapid electrochemical detection of coronavirus SARS-CoV-2. Nat. Commun. 2021, 12, 802.
169. Ramírez-Chavarría, R. G.; Castillo-Villanueva, E.; Alvarez-Serna, B. E.; et al. Loop-mediated isothermal amplification-based electrochemical sensor for detecting SARS-CoV-2 in wastewater samples. J. Environ. Chem. Eng. 2022, 10, 107488.
170. Hussein, H. A.; Hanora, A.; Solyman, S. M.; Hassan, R. Y. A. Designing and fabrication of electrochemical nano-biosensor for the fast detection of SARS-CoV-2-RNA. Sci. Rep. 2023, 13, 5139.
171. Kocheril, P. A.; Lenz, K. D.; Mascareñas, D. D. L.; Morales-Garcia, J. E.; Anderson, A. S.; Mukundan, H. Portable waveguide-based optical biosensor. Biosensors 2022, 12, 195.
172. Bloemen, B.; Gand, M.; Vanneste, K.; Marchal, K.; Roosens, N. H. C.; De Keersmaecker, S. C. J. Development of a portable on-site applicable metagenomic data generation workflow for enhanced pathogen and antimicrobial resistance surveillance. Sci. Rep. 2023, 13, 19656.
173. Awotunde, J. B.; Ogundokun, R. O.; Misra, S. Cloud and IoMT-based big data analytics system during COVID-19 pandemic. In: Chakraborty C, Ghosh U, Ravi V, Shelke Y, editors. Efficient data handling for massive Internet of Medical Things. Cham: Springer International Publishing; 2021. pp. 181-201.
174. Ahila, A.; Dahan, F.; Alroobaea, R.; et al. A smart IoMT based architecture for E-healthcare patient monitoring system using artificial intelligence algorithms. Front. Physiol. 2023, 14, 1125952.
175. Al Nuaimi, D.; Awofeso, N. The value of applying big data analytics in health supply chain management. F1000Res 2024, 13, 1237.
176. Wang, Q.; Su, M.; Zhang, M.; Li, R. Integrating digital technologies and public health to fight COVID-19 pandemic: key technologies, applications, challenges and outlook of digital healthcare. Int. J. Environ. Res. Public. Health. 2021, 18, 6053.
177. El-Deep, S. E.; Abohany, A. A.; Sallam, K. M.; El-Mageed, A. A. A. A comprehensive survey on impact of applying various technologies on the internet of medical things. Artif. Intell. Rev. 2025, 58, 11063.
178. Witteveen-Freidl, G.; Lauenborg Møller, K.; Voldstedlund, M.; Gubbels, S.; Statens Serum Institut COVID-19 Automated Surveillance Group. Data for action - description of the automated COVID-19 surveillance system in Denmark and lessons learnt, January 2020 to June 2024. Epidemiol. Infect. 2025, 153, e58.
179. Frydas, I. S.; Kermenidou, M.; Karypidou, M.; Karakitsios, S.; Sarigiannis, D. A. “SARS-CoV-2 airborne detection within different departments of a COVID-19 hospital building and evaluation of air cleaners in air viral load reduction”. J. Aerosol. Sci. 2025, 187, 106587.
180. Olsen Martinez, A.; Dietz, L. G.; Parhizkar, H.; et al. Air, surface, and wastewater surveillance of SARS-CoV-2; a multimodal evaluation of COVID-19 detection in a built environment. J. Expo. Sci. Environ. Epidemiol. 2025.
181. Li, G.; Diggle, P.; Blangiardo, M. Integrating wastewater and randomised prevalence survey data for national COVID surveillance. Sci. Rep. 2024, 14, 5124.
182. Chaudhuri, A.; Pangaria, A.; Sodhi, C.; et al. Building health system resilience and pandemic preparedness using wastewater-based epidemiology from SARS-CoV-2 monitoring in Bengaluru, India. Front. Public. Health. 2023, 11, 1064793.
183. Rahman, Z.; Liu, W.; Stapleton, L.; et al. Wastewater-based monitoring reveals geospatial-temporal trends for antibiotic-resistant pathogens in a large urban community. Environ. Pollut. 2023, 325, 121403.
184. Erickson, T. B.; Endo, N.; Duvallet, C.; et al. “Waste not, want not” - leveraging sewer systems and wastewater-based epidemiology for drug use trends and pharmaceutical monitoring. J. Med. Toxicol. 2021, 17, 397-410.
186. Rahman, M. T.; Sobur, M. A.; Islam, M. S.; et al. Zoonotic diseases: etiology, impact, and control. Microorganisms 2020, 8, 1405.
187. Li, X.; Zhang, S.; Shi, J.; Luby, S. P.; Jiang, G. Uncertainties in estimating SARS-CoV-2 prevalence by wastewater-based epidemiology. Chem. Eng. J. 2021, 415, 129039.
188. Amereh, F.; Negahban-Azar, M.; Isazadeh, S.; et al. Sewage systems surveillance for SARS-CoV-2: identification of knowledge gaps, emerging threats, and future research needs. Pathogens 2021, 10, 946.
189. Servetas, S. L.; Parratt, K. H.; Brinkman, N. E.; et al. Standards to support an enduring capability in wastewater surveillance for public health: Where are we? Case. Stud. Chem. Environ. Eng. 2022, 6, 100247.
190. Tlhagale, M.; Liphadzi, S.; Bhagwan, J.; et al. Establishment of local wastewater-based surveillance programmes in response to the spread and infection of COVID-19 - case studies from South Africa, the Netherlands, Turkey and England. J. Water. Health. 2022, 20, 287-99.
191. Misra, A.; Powell, E. A. Preanalytical challenges of molecular microbiology tests. Clin. Lab. Med. 2024, 44, 33-43.
192. Khan, M. S.; Dar, O.; Erondu, N. A.; et al. Using critical information to strengthen pandemic preparedness: the role of national public health agencies. BMJ. Global. Health. 2020, 5, e002830.
193. Saini, G.; Deepak, P. S. Wastewater-based epidemiology for novel Coronavirus detection in wastewater. Glob. J. Enviro. Sci. Manage. 2021, 7, 643-58.
194. Ros, F.; Kush, R.; Friedman, C.; et al. Addressing the COVID-19 pandemic and future public health challenges through global collaboration and a data-driven systems approach. Learn. Health. Syst. 2020, 5, e10253.
195. Tosta, S.; Moreno, K.; Schuab, G.; et al. Global SARS-CoV-2 genomic surveillance: what we have learned (so far). Infect. Genet. Evol. 2023, 108, 105405.
196. Chea, T. G.; Quoie, G. D. S. J.; Yang, Y. Application and challenge of wastewater-based epidemiology for the COVID-19 epidemic control in countries at different developing levels. J. Water. Process. Eng. 2024, 58, 104911.
197. Bowes, D. A. Towards a precision model for environmental public health: wastewater-based epidemiology to assess population-level exposures and related diseases. Curr. Epidemiol. Rep. 2024, 11, 131-9.
198. Contreras, V.; Georgeff, V.; Iglesias-Mendoza, G.; et al. Integrating wastewater analysis and targeted clinical testing for early disease outbreak detection and an enhanced public health response. Environ. Sci. Water. Res. Technol. 2025, 11, 317-27.
199. Guilfoyle, R.; Morzycki, A. D.; Saleh, A. What makes global healthcare partnerships successful? A systematic review. Glob. Public. Health. 2022, 17, 662-71.
200. Macedo, H. E.; Lehner, B.; Nicell, J.; et al. Distribution and characteristics of wastewater treatment plants within the global river network. Earth. Syst. Sci. Data. 2022, 14, 559-77.
201. Villar Miguelez, C.; Monzon Baeza, V.; Parada, R.; Monzo, C. Guidelines for renewal and securitization of a critical infrastructure based on IoT networks. Smart. Cities. 2023, 6, 728-43.
202. Murni, I. K.; Oktaria, V.; Handley, A.; et al. The feasibility of SARS-CoV-2 surveillance using wastewater and environmental sampling in Indonesia. PLoS. One. 2022, 17, e0274793.
203. Lott, M. E.; Norfolk, W. A.; Dailey, C. A.; et al. Direct wastewater extraction as a simple and effective method for SARS-CoV-2 surveillance and COVID-19 community-level monitoring. FEMS. Microbes. 2023, 4, xtad004.
204. Wartell, B. A.; Proano, C.; Bakalian, L.; et al. Implementing wastewater surveillance for SARS-CoV-2 on a university campus: lessons learned. Water. Environ. Res. 2022, 94, e10807.
205. Zheng, X.; Wang, M.; Deng, Y.; et al. A rapid, high-throughput, and sensitive PEG-precipitation method for SARS-CoV-2 wastewater surveillance. Water. Res. 2023, 230, 119560.
206. Madimenos, F. C.; Gildner, T. E.; Eick, G. N.; Sugiyama, L. S.; Snodgrass, J. J. Bringing the lab bench to the field: Point-of-care testing for enhancing health research and stakeholder engagement in rural/remote, indigenous, and resource-limited contexts. Am. J. Human. Biol. 2022, 34, e23808.
207. Mei, J.; Wang, D.; Zhang, Y.; et al. Portable paper-based nucleic acid enrichment for field testing. Adv. Sci. 2023, 10, 2205217.
208. Stephens, C. M.; Ho, M.; Schmeidl, S.; et al. International capacity building to achieve SDG6: Insights from longitudinal analysis of five water operator partnerships. Int. J. Water. Resour. Dev. 2023, 39, 557-75.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data





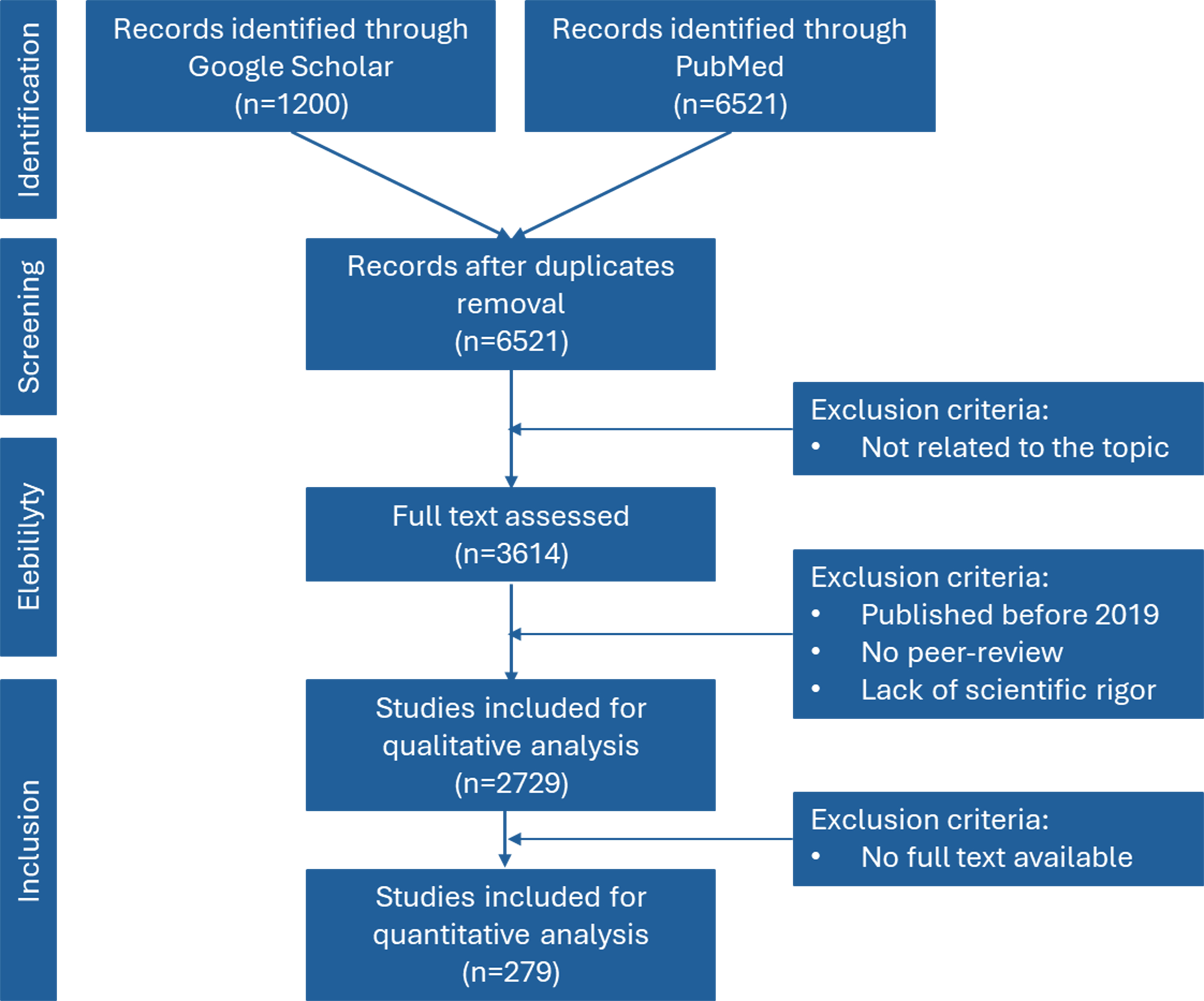















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].