Mildly oxidized and phenol-enriched carbon nanotubes as efficient and selective electrocatalysts for the 2e- oxygen reduction reaction
Abstract
The electrochemical 2e- oxygen reduction reaction (ORR) represents a green, cost-effective strategy towards hydrogen peroxide (H2O2) production other than a promising and more sustainable alternative to the currently anthraquinone-based technology. Light-weight hetero-doped carbon networks, particularly oxidized systems containing variables O-functionalities, have been deeply investigated as promising and selective metal-free electrocatalysts for the process. Following previous and positive outcomes from our team on the tailored surface engineering of complex C-nanocarbon networks with phenolic dangling groups as effective O-functionalities engaged for the molecular oxygen activation and its selective (2e-) electroreduction, we propose hereafter a facile, scalable and highly reproducible one-pot protocol for the mild and controlled oxidation of multi-walled carbon nanotubes. The as-prepared materials have shown a phenolic enriched surface and a superior ability to foster the almost chemoselective 2e- ORR process already under low overpotential values.
Keywords
INTRODUCTION
Hydrogen peroxide (H2O2) is a widespread, environmentally friendly oxidant whose applications span from fine chemical synthesis to paper bleaching and up to medical disinfection[1,2]. Its global demand has reached 4.5 million metric tons in 2020 and it is expected to grow up to 5.7 million metric tons by 2027[3]. Current technologies for H2O2 production mainly rely on the anthraquinone oxidation process that still suffers from many constraints such as: (1) the use of expensive platinum group metals (PGM)-based catalysts; (2) the need of centralized and large-scale infrastructures; (3) the existence of convenient and safe solutions for the H2O2 transportation/delivery to its ultimate destination[4,5].
A valuable and sustainable alternative for the on-site H2O2 production is represented by the partial (2e-) electrochemical reduction of dioxygen[6,7]. Oxygen reduction reaction (ORR) is a multielectron process that occurs through a 2e- or 4e- reduction path. While a complete 4e- reduction to water is widely exploited in the cathodic part of fuel cell devices[8,9], the partial 2e- reduction path can be conveniently employed for
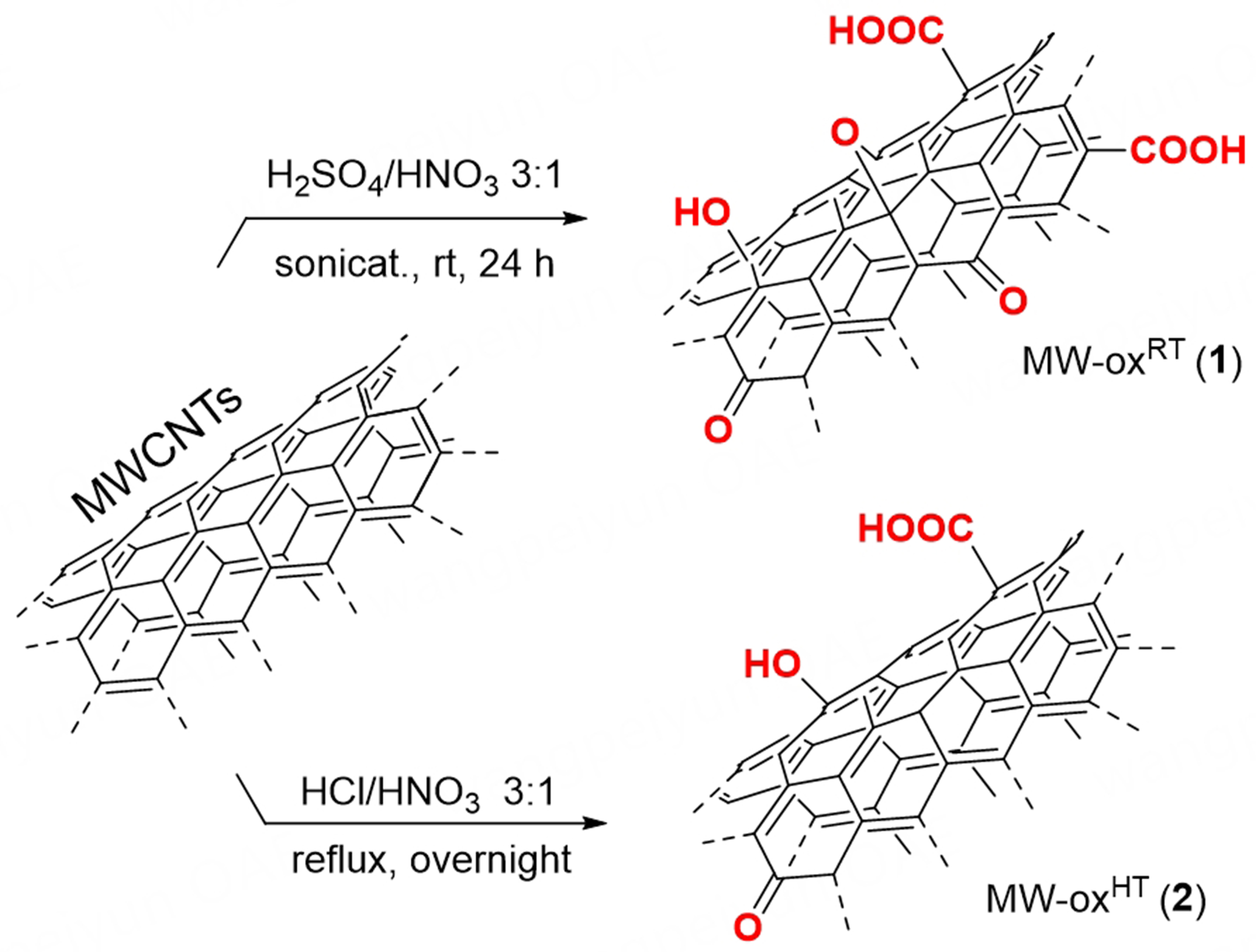
In this work we have reported on two alternative, simple and easily scalable synthetic protocols for the direct surface oxidation of ultra-pure multi-walled carbon nanotubes (MWCNTs, ≥ 98% in C) and their use as metal-free electrocatalysts for the selective 2e- ORR. To this end, two different oxidation schemes for the bulk and multi-gram scale treatment of MWCNTs have been described and oxidation products have been thoroughly analyzed with respect to their electrocatalytic performance in the 2e- ORR. In particular, MW-oxRT (1) was prepared according to the classical sulfo-nitric acid treatment[27,28] commonly exploited to maximize C-materials surface decoration with carboxylic moieties, while an alternative oxidation scheme based on the use of aqua regia was exploited to tune the O-content (oxidation degree) and relative distribution of various O-containing functionalities at the nanomaterial surface [i.e., phenolic vs. carboxylic groups, MW-oxHT (2)]. Our findings have unveiled that oxidation degree of C-networks did not affect linearly ORR activity whereas selectivity towards 2e- ORR was largely boosted by phenol-enriched MWCNTs prepared under milder oxidation conditions based on the use of aqua regia.
EXPERIMENTAL
General considerations
MWCNTs (MWCNTs > 98% in C, Lot# MKBH5814V) and chemicals were provided by Merck and used as received. An Elma S15 Elmasonic sonicator bath (37 kHz) was employed for materials sonication while carbon nanotube (CNT) filtration was accomplished on polytetrafluoroethylene (PTFE) filters (Whatman®, 0.2 μm pore size) upon wetting with alcoholic solutions.
Direct surface oxidation of highly C-pure MWCNTs
A first batch of oxidized MWCNTs was prepared according to a mild (r.t.) oxidation protocol from the literature[27]. In brief, 100 mg of MWCNTs were sonicated in 16 mL of a H2SO4/HNO3 3:1 mixture at room temperature for 24 h. Afterwards, 20 mL of distilled water were added portion-wise and the resulting suspension was collected by filtration through a PTFE membrane. The solid was then washed with distilled water and till complete neutrality of the filtering liquors. The collected oxidized sample MW-oxRT (1) was then dried under vacuum to constant weight and regularly stored on air.
A second oxidation batch of MWCNTs was prepared upon a high-temperature (≈ 108 °C) treatment of the C-network with aqua regia. On this ground, 100 mg of MWCNTs were suspended in 16 mL of a HCl/HNO3 3:1 mixture (aqua regia) and heated overnight at the solvent temperature reflux. Afterwards, the mixture was cooled down to r.t. and treated portion-wise with 20 mL of distilled water before recovering the suspended solid by filtration through a PTFE membrane. Similarly to the above, the solid MW-oxHT (2) was then washed with distilled water and till complete neutrality of the filtering liquors. The oxidized solid was finally dried under vacuum to constant weight and stored at room temperature on air.
Materials characterization and electrochemical analyses
Thermogravimetric analysis (TGA) was performed on an EXSTAR Thermo Gravimetric Analyzer Seiko 6200 in the 40-750 °C range (5 °C/min) under N2 atmosphere (100 mL/min). Elemental analysis (EA) was accomplished by means of a Thermo FlashEA 1112 Series CHNS analyzer. Fourier-transform infrared (FT-IR) analyses were performed on a PerkinElmer Spectrum BX spectrophotometer in the 400-4,000 cm-1 range and with a resolution of 1 cm-1. Nitrogen physisorption analyses were conducted on a Micromeritics ASAP 2020 instrument at 77 K after degassing the samples for 24 h at 120 °C. Specific surface area (SSA) was calculated by the Brunauer-Emmet-Teller (BET) while pore size distribution was determined by Barrett-Joyner-Halenda (BJH) method. Total pore volume was estimated at p/p0 = 0.98 relative pressure. X-ray photoelectron spectroscopy (XPS) was performed in an ultra-high vacuum system (10-9/10-10 mbar). The chamber was equipped with non-monochromatized dual anode (Al and Mg) and a hemispherical electron/ion energy analyzer. The operating power of the Al X-ray source was 120 W and photoelectrons were collected normal to the sample surface, maintaining the analyzer angle in the range between analyzer axis and X-ray source fixed at 54.5°. The spectra were elaborated using CasaXPS software[29] and peaks were deconvoluted using mixed Gaussian and Lorentzian functions. All spectra were calibrated on the basis of the C1s sp2 component fixed at 284.8 eV[25]. Samples for analyses were dispersed in ethanol upon sonication and drop cast on a gold plate to avoid any C or O contamination from the analyses support. High resolution transmission electron microscopy (TEM) was carried out on a Thermo-Fisher F200X G2 HR-TEM
Electrochemical measurements were performed by means of a rotating ring-disk electrode (RRDE) from Pine Instrument Co. composed by a glassy carbon (GC) disk (A = 0.2376 cm2) and a Pt ring (A =
H2O2 selectivity was estimated either from Pt ring currents [Equation (1)] of the RRDE electrode and from the average number of exchanged electrons (n) per O2 molecule as obtained by Koutecky-Levich equation [Equation (2)]:
where IR and ID are the ring and the disk current values, respectively, and N is the ring collection efficiency. The latter parameter was determined experimentally using a standard K3Fe(CN)6 solution in KCl (N = 0.23).
where J is the current density (mA/cm2), JK is the kinetic current density (mA/cm2), n is the number of exchanged electrons, F is the Faraday constant, C0 is the O2 concentration in solution (1.15 × 10-3 mol/dm3), D0 is the oxygen diffusion coefficient (1.95 × 10-5 cm2/s), ν is the electrolyte kinematic viscosity
RESULTS AND DISCUSSION
Synthesis of oxidized MW-oxRT (1) and MW-oxHT (2) samples
Two alternative, one-pot oxidation protocols have been applied to the preparation of oxidized MWCNTs [Scheme 1].
MW-oxRT (1) was prepared according to classical procedures from the literature aimed at maximizing the generation of O-containing functionalities at the nanomaterial surface[27,28]. In particular, highly C-pure MWCNTs dispersed in a concentrated sulfuric and nitric acid mixture have been oxidized at ambient temperature and pressure in a sonicator bath (37 kHz) for 24 h (see Experimental Section for details). This approach has extensively been employed to maximize the loading of carboxylic acid groups at the outer surface of CNTs, thus enhancing their hydrophilic character. If the latter feature is frequently invoked as a key pre-requisite of carbon-based nanomaterial as electrocatalysts in water-based electrolytes[36], recent work by our group has unveiled that carboxylic acid groups do not generate the ideal electronic microenvironment to promote the 2e- ORR efficiently[25]. Despite the authors’ awareness of the often batch-dependent nature of CNT samples prepared through straightforward bottom-up oxidation methods - as well as the limited control over the chemical composition and loading of oxygen-containing functional groups in the oxidized nanomaterials - we compared these preliminary, mild oxidation conditions with more severe ones. This comparison was made without overlooking the reproducibility of newly oxidized batches and their ultimate electrocatalytic performance in the ORR. The aim was to select the most appropriate batch conditions for a practical and easily scalable production of oxidized CNT samples featuring an optimal balance between loading and chemical identity of surface O-containing groups thus creating ideal surface microenvironments to boost the metal-free 2e- ORR selectively. To this aim a second oxidized C-sample [MW-oxHT (2)] was prepared without the aid of ultrasounds (that are known to maximize O-content)[37] by refluxing overnight a CNT dispersion in aqua regia. A similar treatment of CNTs has already been reported in the literature but at room temperature with the production of only moderately wettable oxidized-CNTs samples[28].
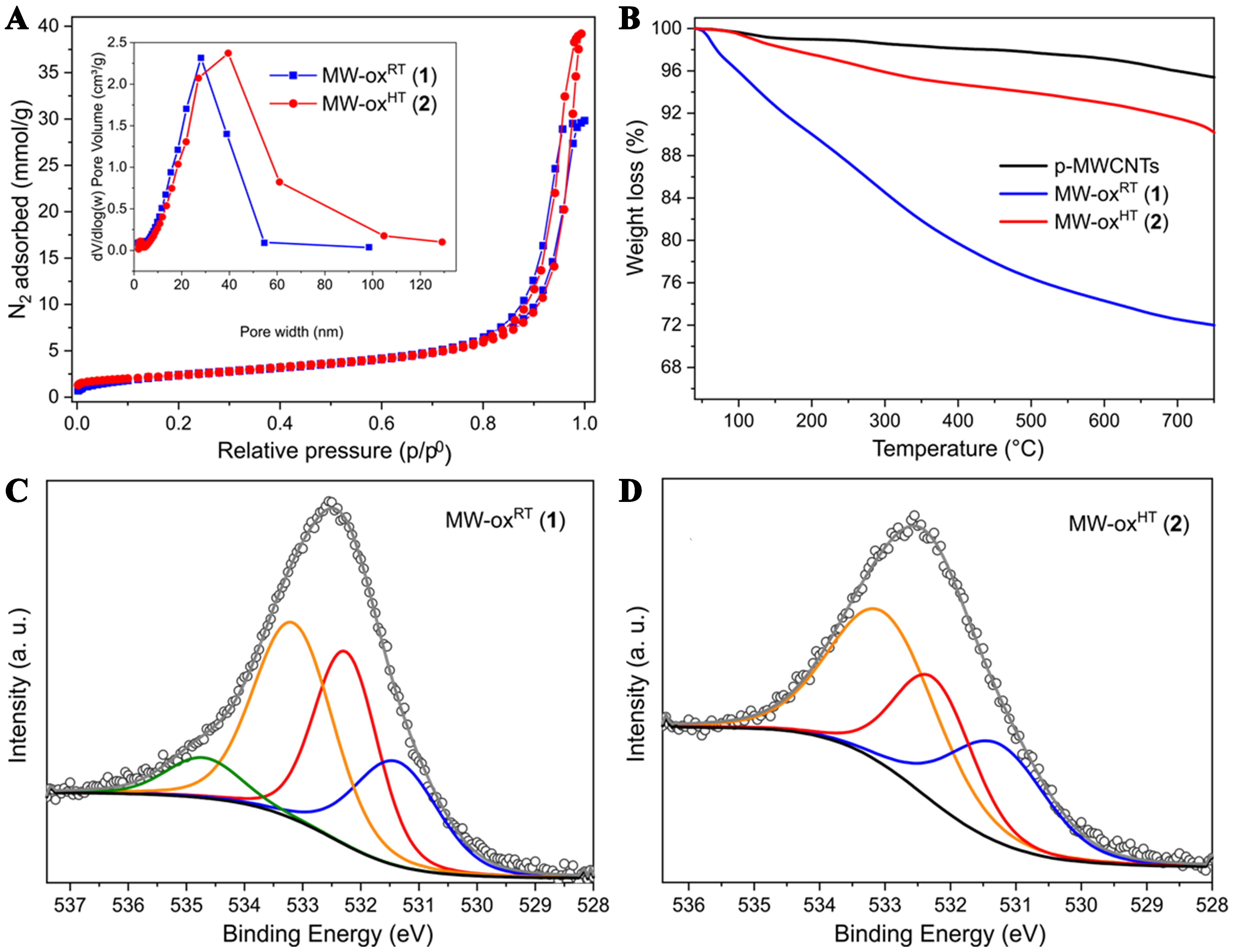
The two oxidized samples have been thoroughly characterized before being employed as electrocatalysts for the 2e- ORR. From a morphological viewpoint, TEM images recorded from evaporative casting of alcoholic suspensions of MW-oxRT (1) and MW-oxHT (2) do not show relevant difference [Supplementary Figure 1], except for a superior aggregation degree of C-flakes in MW-oxHT (2). This is in accord with the lower oxidation degree measured in 2 (vide infra). Nitrogen physisorption analyses were carried out on the MWCNT sample before and after both oxidation protocols. Samples SSA showed largely superimposable BET profiles with only minor alterations of total pore volumes [p-MWCNTs 183 m2/g and 0.94 cm3/g; MW-oxRT (1) 203 m2/g and 0.95 cm3/g; MW-oxHT (2) 190 m2/g and 1.06 cm3/g]. All materials exhibit type IV isotherms featured by small H2 hysteresis loops in the 0.8 ÷ 1.0 p/p0 range typical of mesoporous networks
Figure 1. (A) N2 adsorption-desorption isothermal curves conducted at 77 K for MW-oxRT (1) and MW-oxHT (2) along with their corresponding pore size distribution (inset); (B) TGA conducted on MW-oxRT (1) and MW-oxHT (2) in comparison with their pristine counterpart. Analyses conditions: from 40 to 750 °C (5 °C/min) in N2 atmosphere (100 mL/min); (C) High-resolution O 1s XPS signal of MW-oxRT (1) and (D) MW-oxHT (2) along with their fitting deconvolution. TGA: Thermogravimetric analysis; XPS: X-ray photoelectron spectroscopy.
Raman spectroscopy carried out on samples 1 and 2 reflects their different oxidation degree. As Supplementary Figure 4 shows, both oxidized samples exhibit higher intensity ratios of D and G modes
TGA, EA and high-resolution O 1s XPS spectroscopy have finally been employed to get more details on the chemico-physical composition of 1 and 2. Comparative TGA profiles of pristine MWCNTs along with those of oxidized samples 1 and 2 are provided on Figure 1B. Both oxidized samples show distinctive weight losses ascribed to the presence of thermally labile O-containing functionalities. This comparative analysis also highlights the significant gap between the two oxidized samples in terms of surface loading of O-containing functional groups as a consequence of the different oxidation protocols to get 1 and 2. Accordingly, 1 exhibits a markedly superior weight loss in the 40-750 °C thermal region compared to sample 2 thus confirming the superior ability of sulfo-nitric acid mixture to deeply oxidize nanocarbon materials already under mild (r.t.) temperature conditions[27,28]. The different functionalization degrees of samples 1 and 2 have also been confirmed by EA and XPS analyses [Supplementary Table 1]. EAs clearly indicate a lower C-content in MW-oxRT (1) with respect to MW-oxHT (2), thus indirectly pointing out the superior oxidation degree of the former sample. On the same ground, XPS survey spectra [Supplementary Figure 5] show a lower C/O signals ratio in 1 vs. 2. To investigate the nature of O-containing groups in the oxidized materials, high resolution O 1s XPS signals were deconvoluted into three main components: 531.3 ± 0.2, 532.3 ± 0.2 and 533.1 ± 0.2 eV ascribed to carbonyl, carboxylic acids and C-OH groups (from carboxylic acids and phenols), respectively[25,41,42]. As shown in Figure 1C and summarized in Supplementary Table 1, MW-oxRT (1) presents a markedly high content of carboxylic groups (> 50%). On the other hand, the oxidation treatment at higher temperature values with aqua regia gives rise to materials oxidized at a lower extent but featuring a higher percentage of phenol-like moieties [Figure 1D and Supplementary Table 1]. A minor O 1s XPS component at 534.7 eV in 1 is finally ascribed to adventitious water[43] [Figure 1C], as a consequence of the superior hydrophilic character of this highly oxidized sample.
Electrocatalytic study of 1 and 2 in the 2e- ORR
MW-oxRT (1) and MW-oxHT (2) have been tested as electrocatalysts for the selective 2e- ORR to hydrogen peroxide. To this aim, all powdery C-based samples were dispersed upon sonication in a water/alcohol/Nafion® solution before being drop cast on a GC disk of the RRDE and left to evaporate at ambient conditions to get ultra-thin C-coatings (working electrode, see Experimental part for details). Electrochemical measurements were then carried out in an O2-saturated alkaline solution using a three electrodes cell including - other than the working electrode - a Pt wire and an Ag/AgCl/KClsat. as counter and reference electrodes, respectively.
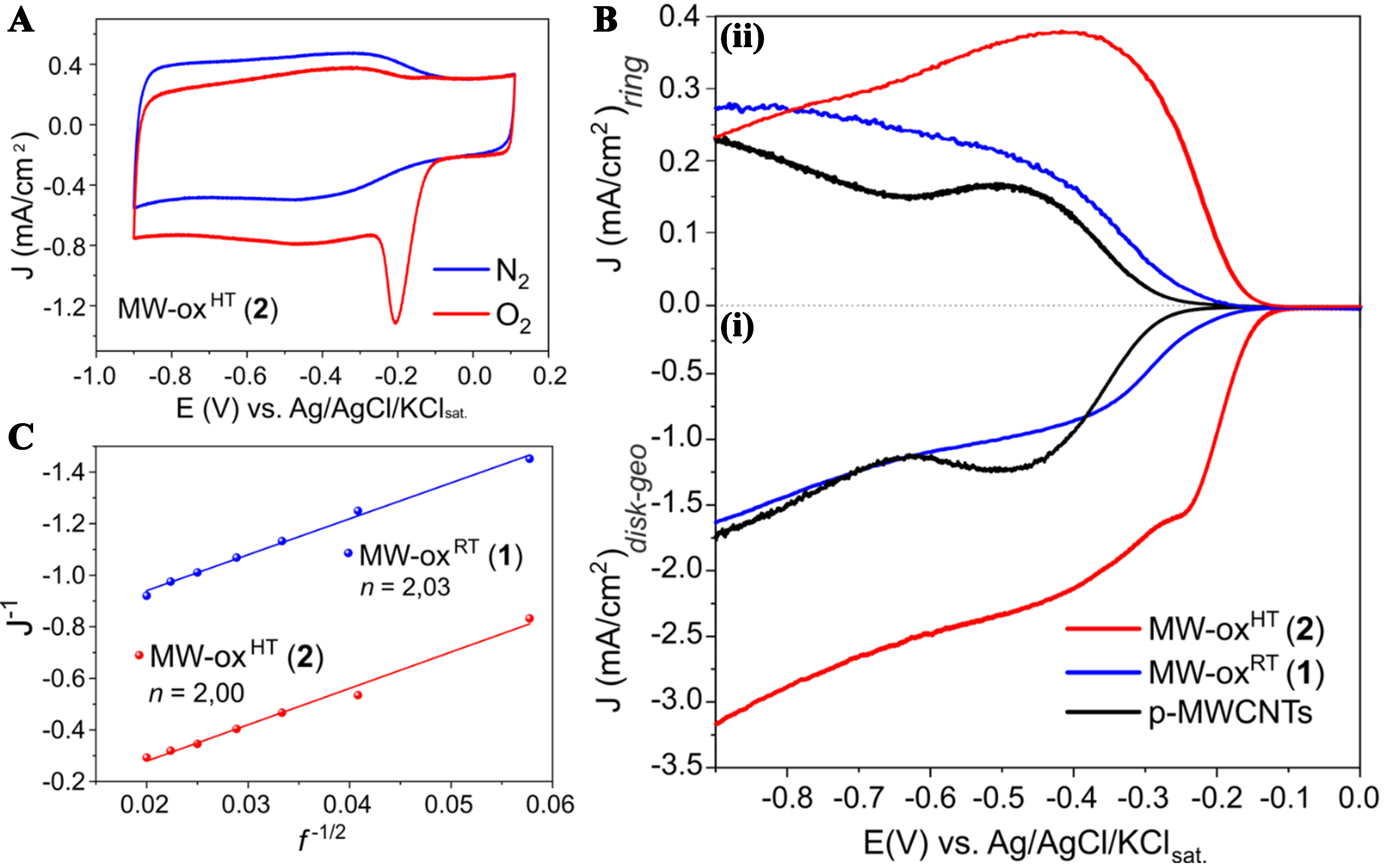
Qualitative evidence of the materials activity in ORR has first been achieved from cyclic voltametric tests. As shown in Supplementary Figure 6 and Figure 2A, both samples exhibit a well-defined reduction peak under O2-saturated conditions not observed in N2-saturated environments. Linear sweep voltammograms (LSV) have finally been acquired in the +0.1 ÷ -0.9 V range at an electrode spin rate of 900 rpm to get further details on the ORR activity of oxidized samples 1 and 2 along with that of their pristine counterpart (MWCNTs). Background currents measured under saturated N2 conditions were then subtracted from each curve to eliminate the capacitive contributions. As shown in Figure 2B(i), both oxidized materials display positive onset overpotential shifts compared with pristine MWCNTs. In particular, MW-oxHT (2) shows an onset value of -0.14 V vs. Ag/AgCl/KClsat. [Table 1], with a remarkable overpotential shift of about +160 mV with respect to pristine MWCNTs.
Figure 2. (A) CV acquired in N2 and O2 saturated KOH 0.1 M solution for MW-oxHT (2) at 10 mV/s in the +0.1 ÷ -0.9 V vs. Ag/AgCl/KClsat. potential range; [B(i)] LSV curves acquired for p-MWCNTs, MW-oxRT (1) and MW-oxHT (2) in O2-saturated KOH 0.1 M solution at 900 rpm at the GC disk electrode and [B(ii)] correspondent curves registered at the Pt-ring electrode; (C) K-L plot of MW-oxRT (1) and MW-oxHT (2) extrapolated from respective LSV curves at -0.4 V. CV: Cyclic voltammograms; LSV: linear sweep voltammograms; MWCNTs: multi-walled carbon nanotubes; GC: glassy carbon.
Onset and half-wave potential values, H2O2%, average number of exchanged electrons (n) per O2 molecule and mass activity measured for p-MWCNTs, MW-oxRT (1) and MW-oxHT (2)
| Sample | EON (V)a | E1/2 (V)a | H2O2 (%)b | n K-L c | Mass activity (mA/mgCAT)d |
| p-MWCNTs | -0.30 | -0.39 | 50 | - | 3.21 |
| MW-oxRT (1) | -0.20 | -0.38 | 87 | 2.03 | 2.93 |
| MW-oxHT (2) | -0.14 | -0.25 | 85 | 2.00 | 7.25 |
In addition to a markedly low onset potential value, MW-oxHT (2) exhibits a pretty high Pt-ring current density [Figure 2B(ii)] directly related to the amount of hydrogen peroxide produced. As a matter of fact, a 2e- dioxygen reduction pathway with a selectivity up to 85% [Table 1 and Supplementary Figure 7A] is observed with this oxidized material at work. In spite of an equally high selectivity of 1 towards H2O2, the markedly lower current density and the worse onset potential value (-0.20 V) of this electrocatalyst, make it a less attractive material for the selective 2e- ORR [Table 1]. The Koutecky-Levich equation has finally been applied to measure the average number of electrons transferred per O2 molecule (n) with the two electrocatalysts at comparison. To this end, LSV curves of each sample have been recorded at different electrode spin rates (from 300 to 2,500 rpm, Supplementary Figure 8) and the corresponding K-L plots have traced out [Figure 2C]. Apart from the limits of analytical methods applied to the quantification of H2O2[44], K-L plots have confirmed the ability of both oxidized samples to foster a prevalent 2e- transfer process, typical of a chemoselective O2 reduction path to hydrogen peroxide [Table 1 and Supplementary Figure 7B]. As mentioned above, MW-oxHT (2) also presents a net superior current density with respect to MW-oxRT (1) counterpart as confirmed by the higher half-wave potential value of the former [Figure 2B(i) and Table 1]. As a consequence, the H2O2 mass activity expressed as mA per mgCAT is more than doubled in 2 vs. 1 and/or their pristine counterpart MWCNTs [Table 1]. Tafel analysis further confirms the superior catalytic activity of MW-oxHT (2). As illustrated in Supplementary Figure 9, 2 exhibits a significantly lower Tafel slope of
Close selectivity values of the two oxidized samples led us to conclude that whatever the electrocatalyst at work (1 or 2), after dioxygen adsorption [Equation (3)] and the first proton-coupled electron transfer [Equation (4)], both materials bind the key intermediate HOO* weakly enough to allow its fast desorption [Equation (5), right-hand side] before O-O dissociation path [Equation (5), left-hand side and Equation (6)] takes place (competitive 4e- ORR).
On the other hand, the superior mass-activity with 2 is basically attributed to unique surface chemico-physical properties of the sample, as the result of an unconventional and high-temperature oxidation treatment of MWCNTs with aqua regia. The lower oxidation degree (i.e., the reduced loading of O-containing functional groups), combined with the variable chemical composition of surface groups (i.e., phenolic vs. carboxylic groups), is supposed to generate an ideal and highly reproducible surface microenvironment for the efficient and low-overpotential activation/reduction of dioxygen.
Lastly, the net improvement of 2e- ORR performance (activity and selectivity) measured in the sequence pristine MWCNTs vs. 1 vs. 2 [Table 1] demonstrate the key role of selected O-containing functionalities (i.e., phenol groups vs. carboxylic acids) on the electroreduction path, rather than the material oxidation degree. Neither a too high oxidation degree of the nanomaterial nor the presence of a relatively higher concentration of carboxylic acid at its outer surface [i.e., sulfo-nitric vs. chloro-nitric (aqua regia) oxidation method], seem to really control the 2e- activation/reduction of dioxygen.
CONCLUSIONS
Two oxidized MWCNTs materials have been straightforwardly prepared using easily scalable and highly reproducible synthetic protocols. MW-oxRT (1) and MW-oxHT (2) isolated from the purification of MWCNT suspensions in sulfo-nitric or chloro-nitric (aqua regia) mixtures, respectively, have been characterized with respect to their morphological and chemical composition before being tested as metal-free electrocatalysts for the challenging 2e- ORR process. The sample prepared by the aqua regia oxidation method [MW-oxHT
Guided by our results on 2e- ORR promoted by tailored O-decorated carbon nanomaterials[25], a facile, scalable and highly reproducible one-pot protocol has now been developed to generate moderately oxidized MWCNTs containing relatively high concentrations of surface phenolic groups. The latter have confirmed their key role in fostering the selective and efficient 2e- reduction of dioxygen already under low overpotential values.
DECLARATIONS
Authors’ contributions
Conceptualization: Tuci, G.; Bonechi, M.; Innocenti, M.; Giambastiani, G.
Methodology and its development: Tuci, G.; Giambastiani, G.
Validation: Tuci, G.; Giambastiani, G.; Bonechi, M.
Formal analysis: Tuci, G.; Bonechi, M.; Rossin, A.; Berretti, E.; Ceppatelli, M.; Poggini, L.
Investigation: Tuci, G.; Bonechi, M.
Data curation: Tuci, G.; Giambastiani, G.; Bonechi, M.; Innocenti, M.; Ceppatelli, M.
Writing - original draft: Tuci, G.; Giambastiani, G.; Bonechi, M.
Supervision: project management/coordination: Tuci, G.; Giambastiani, G.; Innocenti, M.
Funding acquisition: Tuci, G.; Giambastiani, G.; Innocenti, M.
Availability of data and materials
The raw data supporting the findings of this study are available within this Article and its Supplementary Materials. Further data is available from the corresponding authors upon reasonable request.
Financial support and sponsorship
The authors would like to thank the Italian MUR through the PRIN2022 project “MATISSE - A ‘Molecular Lift’ for the Control of the Metal Protrusion and Coordination Sphere in Single-Atom Catalysts for CO2 Electroreduction” (2022K5SX27) and the European Union - NextGeneration EU through the Italian Ministry of Environment and Energy Security POR H2 AdP MMES/ENEA with involvement of CNR and RSE, PNRR - Mission 2, Component 2, Investment 3.5 “Ricerca e sviluppo sull’idrogeno”, CUP: B93C22000630006 for financial support to this work. The financial support provided by the MUR - Dipartimenti di Eccellenza 2023-2027 (DICUS 2.0) to the Department of Chemistry “Ugo Schiff” of the University of Florence is also acknowledged. Acknowledgements are expressed to the European Union - NextGeneration EU for funding the project IPHOQS “Integrated Infrastructure Initiative in Photonic and Quantum Sciences” - I-PHOQS [IR0000016, ID D2B8D520, CUP B53C22001750006] and the Circular and Sustainable Made in Italy Extended Partnership (MICS), Piano Nazionale di Ripresa e Resilienza (PNRR) - Missione 4, Componente 2, Investimento 1.3 - D.D. 1551.11-10-2022, PE00000004.
Conflicts of interest
Giambastiani, G. is Guest Editor of the Special Issue “Carbon in Catalysis” and Associate Editor of the journal Chemical Synthesis. Giambastiani, G. was not involved in any steps of editorial processing, notably including reviewers’ selection, manuscript handling, or decision-making. The other authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Targhan, H.; Evans, P.; Bahrami, K. A review of the role of hydrogen peroxide in organic transformations. J. Ind. Eng. Chem. 2021, 104, 295-332.
2. Fukuzumi, S.; Lee, Y.; Nam, W. Recent progress in production and usage of hydrogen peroxide. Chin. J. Catal. 2021, 42, 1241-52.
3. Lee, K.; Lim, J.; Lee, M. J.; et al. Structure-controlled graphene electrocatalysts for high-performance H2O2 production. Energy. Environ. Sci. 2022, 15, 2858-66.
4. Campos-Martin, J. M.; Blanco-Brieva, G.; Fierro, J. L. Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. Engl. 2006, 45, 6962-84.
5. Yang, S.; Verdaguer-Casadevall, A.; Arnarson, L.; et al. Toward the decentralized electrochemical production of H2O2: a focus on the catalysis. ACS. Catal. 2018, 8, 4064-81.
6. Perry, S. C.; Pangotra, D.; Vieira, L.; et al. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 2019, 3, 442-58.
7. Pang, Y.; Xie, H.; Sun, Y.; Titirici, M.; Chai, G. Electrochemical oxygen reduction for H2O2 production: catalysts, pH effects and mechanisms. J. Mater. Chem. A. 2020, 8, 24996-5016.
8. Zhang, Y. L.; Goh, K.; Zhao, L.; et al. Advanced non-noble materials in bifunctional catalysts for ORR and OER toward aqueous metal-air batteries. Nanoscale 2020, 12, 21534-59.
9. Li, Y.; Li, Q.; Wang, H.; Zhang, L.; Wilkinson, D. P.; Zhang, J. Recent progresses in oxygen reduction reaction electrocatalysts for electrochemical energy applications. Electrochem. Energ. Rev. 2019, 2, 518-38.
10. Papanikolaou, G.; Centi, G.; Perathoner, S.; Lanzafame, P. Catalysis for e-chemistry: need and gaps for a future de-fossilized chemical production, with focus on the role of complex (direct) syntheses by electrocatalysis. ACS. Catal. 2022, 12, 2861-76.
11. Verdaguer-Casadevall, A.; Deiana, D.; Karamad, M.; et al. Trends in the electrochemical synthesis of H2O2: enhancing activity and selectivity by electrocatalytic site engineering. Nano. Lett. 2014, 14, 1603-8.
12. Jirkovský, J. S.; Panas, I.; Ahlberg, E.; Halasa, M.; Romani, S.; Schiffrin, D. J. Single atom hot-spots at Au-Pd nanoalloys for electrocatalytic H2O2 production. J. Am. Chem. Soc. 2011, 133, 19432-41.
13. Wu, S.; Yu, L.; Wen, G.; Xie, Z.; Lin, Y. Recent progress of carbon-based metal-free materials in thermal-driven catalysis. J. Energy. Chem. 2021, 58, 318-35.
14. Jaryal, V. B.; Villa, A.; Gupta, N. Metal-free carbon-based nanomaterials: insights from synthesis to applications in sustainable catalysis. ACS. Sustainable. Chem. Eng. 2023, 11, 14841-65.
15. Tuci, G.; Pilaski, M.; Ba, H.; et al. Unraveling surface basicity and bulk morphology relationship on covalent triazine frameworks with unique catalytic and gas adsorption properties. Adv. Funct. Mater. 2017, 27, 1605672.
16. Yang, L.; Shui, J.; Du, L.; et al. Carbon-based metal-free ORR electrocatalysts for fuel cells: past, present, and future. Adv. Mater. 2019, 31, e1804799.
17. Li, Y.; Tong, Y.; Peng, F. Metal-free carbocatalysis for electrochemical oxygen reduction reaction: activity origin and mechanism. J. Energy. Chem. 2020, 48, 308-21.
18. He, H.; Liu, S.; Liu, Y.; et al. Review and perspectives on carbon-based electrocatalysts for the production of H2O2 via two-electron oxygen reduction. Green. Chem. 2023, 25, 9501-42.
19. Sang, Z.; Hou, F.; Wang, S.; Liang, J. Research progress on carbon-based non-metallic nanomaterials as catalysts for the two-electron oxygen reduction for hydrogen peroxide production. New. Carbon. Mater. 2022, 37, 136-51.
20. Zhou, W.; Xie, L.; Gao, J.; et al. Selective H2O2 electrosynthesis by O-doped and transition-metal-O-doped carbon cathodes via O2 electroreduction: a critical review. Chem. Eng. J. 2021, 410, 128368.
21. Shen, X.; Wang, Z.; Guo, H.; Lei, Z.; Liu, Z.; Wang, L. Solvent engineering of oxygen-enriched carbon dots for efficient electrochemical hydrogen peroxide production. Small 2023, 19, e2303156.
22. Sun, F.; Yang, C.; Qu, Z.; et al. Inexpensive activated coke electrocatalyst for high-efficiency hydrogen peroxide production: coupling effects of amorphous carbon cluster and oxygen dopant. Appl. Catal. B. Environ. 2021, 286, 119860.
23. Zhang, D.; Tsounis, C.; Ma, Z.; et al. Highly selective metal-free electrochemical production of hydrogen peroxide on functionalized vertical graphene edges. Small 2022, 18, e2105082.
24. Tuci, G.; Rossin, A.; Saki, Z.; et al. The still elusive role of lightweight doping in carbon-based electrocatalysts for the selective oxygen reduction reaction to hydrogen peroxide. ChemSusChem 2024, 17, e202400660.
25. Tuci, G.; Rossin, A.; Zhang, X.; et al. Metal-free electrocatalysts for the selective 2e- oxygen reduction reaction: a never-ending story? Chemistry 2023, 29, e202301036.
26. Tuci, G.; Rossin, A.; Zhang, X.; Pham-Huu, C.; Giambastiani, G. Exohedrally functionalized carbon-based networks as catalysts for electrochemical syntheses. Curr. Opin. Green. Sustain. Chem. 2022, 33, 100579.
27. Samorì, C.; Sainz, R.; Ménard-Moyon, C.; et al. Potentiometric titration as a straightforward method to assess the number of functional groups on shortened carbon nanotubes. Carbon 2010, 48, 2447-54.
28. Lavagna, L.; Bartoli, M.; Suarez-Riera, D.; Cagliero, D.; Musso, S.; Pavese, M. Oxidation of carbon nanotubes for improving the mechanical and electrical properties of oil-well cement-based composites. ACS. Appl. Nano. Mater. 2022, 5, 6671-8.
29. Fairley, N.; Fernandez, V.; Richard-Plouet, M.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112.
30. Ceppatelli, M.; Gorelli, F. A.; Haines, J.; Santoro, M.; Bini, R. Probing high-pressure reactions in heterogeneous materials by Raman spectroscopy. Z. Kristallogr. 2014, 229, 83-91.
31. Ferrari, A. C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B. 2000, 61, 14095-107.
32. Ferrari, A. C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B. 2001, 64, 075414.
33. Lucchese, M.; Stavale, F.; Ferreira, E. M.; et al. Quantifying ion-induced defects and Raman relaxation length in graphene. Carbon 2010, 48, 1592-7.
34. Wang, Y.; Zhang, D.; Liu, H. A study of the catalysis of cobalt hydroxide towards the oxygen reduction in alkaline media. J. Power. Sources. 2010, 195, 3135-9.
35. Chen, Y.; Wang, S.; Li, Z. A cobalt-pyrrole coordination compound as high performance cathode catalyst for direct borohydride fuel cells. RSC. Adv. 2020, 10, 29119-27.
36. Dong, K.; Liang, J.; Wang, Y.; et al. Honeycomb carbon nanofibers: a superhydrophilic O2-entrapping electrocatalyst enables ultrahigh mass activity for the two-electron oxygen reduction reaction. Angew. Chem. Int. Ed. Engl. 2021, 60, 10583-7.
37. Jun, L. Y.; Mubarak, N.; Yon, L. S.; Bing, C. H.; Khalid, M.; Abdullah, E. Comparative study of acid functionalization of carbon nanotube via ultrasonic and reflux mechanism. J. Environ. Chem. Eng. 2018, 6, 5889-96.
38. Aranovich, G.; Donohue, M. Analysis of adsorption isotherms: lattice theory predictions, classification of isotherms for gas–solid equilibria, and similarities in gas and liquid adsorption behavior. J. Colloid. Interface. Sci. 1998, 200, 273-90.
39. de Menezes, B. R. C.; Ferreira, F. V.; Silva, B. C.; et al. Effects of octadecylamine functionalization of carbon nanotubes on dispersion, polarity, and mechanical properties of CNT/HDPE nanocomposites. J. Mater. Sci. 2018, 53, 14311-27.
40. Mishra, S. K.; Tripathi, S. N.; Choudhary, V.; Gupta, B. D. Surface plasmon resonance-based fiber optic methane gas sensor utilizing graphene-carbon nanotubes-poly(methyl methacrylate) hybrid nanocomposite. Plasmonics 2015, 10, 1147-57.
41. Yu, B.; Wang, X.; Qian, X.; et al. Functionalized graphene oxide/phosphoramide oligomer hybrids flame retardant prepared via in situ polymerization for improving the fire safety of polypropylene. RSC. Adv. 2014, 4, 31782.
42. Stevens, J. S.; Seabourne, C. R.; Jaye, C.; Fischer, D. A.; Scott, A. J.; Schroeder, S. L. Incisive probing of intermolecular interactions in molecular crystals: core level spectroscopy combined with density functional theory. J. Phys. Chem. B. 2014, 118, 12121-9.
43. Rojas, J.; Toro-Gonzalez, M.; Molina-Higgins, M.; Castano, C. Facile radiolytic synthesis of ruthenium nanoparticles on graphene oxide and carbon nanotubes. Mater. Sci. Eng. B. 2016, 205, 28-35.
44. Zhou, R.; Zheng, Y.; Jaroniec, M.; Qiao, S. Determination of the electron transfer number for the oxygen reduction reaction: from theory to experiment. ACS. Catal. 2016, 6, 4720-8.
45. Lu, Z.; Chen, G.; Siahrostami, S.; et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 2018, 1, 156-62.
46. Kim, H. W.; Ross, M. B.; Kornienko, N.; et al. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts. Nat. Catal. 2018, 1, 282-90.
47. Han, G. F.; Li, F.; Zou, W.; et al. Building and identifying highly active oxygenated groups in carbon materials for oxygen reduction to H2O2. Nat. Commun. 2020, 11, 2209.
48. San Roman, D.; Krishnamurthy, D.; Garg, R.; et al. Engineering three-dimensional (3D) out-of-plane graphene edge sites for highly selective two-electron oxygen reduction electrocatalysis. ACS. Catal. 2020, 10, 1993-2008.
49. Pang, Y.; Wang, K.; Xie, H.; Sun, Y.; Titirici, M.; Chai, G. Mesoporous carbon hollow spheres as efficient electrocatalysts for oxygen reduction to hydrogen peroxide in neutral electrolytes. ACS. Catal. 2020, 10, 7434-42.
50. Chen, S.; Luo, T.; Chen, K.; et al. Chemical identification of catalytically active sites on oxygen-doped carbon nanosheet to decipher the high activity for electro-synthesis hydrogen peroxide. Angew. Chem. Int. Ed. Engl. 2021, 60, 16607-14.
51. Lee, J.; Lee, Y.; Lim, J. S.; et al. Discriminating active sites for the electrochemical synthesis of H2O2 by molecular functionalisation of carbon nanotubes. Nanoscale 2022, 15, 195-203.
52. Wang, W.; Hu, Y.; Liu, Y.; Zheng, Z.; Chen, S. Self-powered and highly efficient production of H2O2 through a Zn-air battery with oxygenated carbon electrocatalyst. ACS. Appl. Mater. Interfaces. 2018, 10, 31855-9.
53. Zhang, H.; Li, Y.; Zhao, Y.; Li, G.; Zhang, F. Carbon black oxidized by air calcination for enhanced H2O2 generation and effective organics degradation. ACS. Appl. Mater. Interfaces. 2019, 11, 27846-53.
54. Zhu, J.; Xiao, X.; Zheng, K.; et al. KOH-treated reduced graphene oxide: 100% selectivity for H2O2 electroproduction. Carbon 2019, 153, 6-11.
55. Sa, Y. J.; Kim, J. H.; Joo, S. H. Active edge-site-rich carbon nanocatalysts with enhanced electron transfer for efficient electrochemical hydrogen peroxide production. Angew. Chem. Int. Ed. Engl. 2019, 58, 1100-5.
56. Lim, J. S.; Kim, J. H.; Woo, J.; et al. Designing highly active nanoporous carbon H2O2 production electrocatalysts through active site identification. Chem 2021, 7, 3114-30.
57. She, F.; Guo, Z.; Liu, F.; et al. Curvature-dependent electrochemical hydrogen peroxide synthesis performance of oxidized carbon nanotubes. ACS. Catal. 2024, 14, 10928-38.
58. Xing, Z.; Shi, K.; Parsons, Z. S.; Feng, X. Interplay of active sites and microenvironment in high-rate electrosynthesis of H2O2 on doped carbon. ACS. Catal. 2023, 13, 2780-9.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].