Outcomes after pelvic fascia-sparing robot-assisted radical prostatectomy vs. standard robot-assisted radical prostatectomy
Abstract
Urinary incontinence immediately following robotic-assisted laparoscopic radical prostatectomy can significantly impact quality of life. Pelvic fascia-sparing robotic-assisted radical prostatectomy (PFS-RARP) was first described in 2010 to improve urinary functional outcomes via further preservation of anterior pelvic fascial structures. In this article, we summarize the anatomic basis, origin, and outcomes of PFS-RARP compared to standard RARP (S-RARP), highlighting potential advantages in urinary continence and ongoing debate over oncologic efficacy.
Keywords
INTRODUCTION
Radical prostatectomy (RP) is the standard treatment for localized prostate cancer, and is the initial treatment of choice for approximately 40% of patients diagnosed with prostate cancer[1]. However, RP has been associated with significant consequences to patients’ quality of life, including urinary incontinence and impaired sexual function[2,3]. Surgical advances have led to minimally invasive surgical techniques, and it is currently estimated that 90% of RPs are performed robotically[1]. Patients undergoing minimally invasive surgery typically have decreased blood loss and faster recovery compared to those undergoing an open approach[4,5]. At its best, RP aims to achieve a trifecta: cancer-free, continent, and potent[6]. While standard robot-assisted radical prostatectomy (S-RARP) has been shown to have higher early continence rates than retropubic radical prostatectomy (RRP) and laparoscopic radical prostatectomy (LRP)[7], urinary incontinence remains one of the most pervasive complications of RP with high associated morbidity and patient bother.
Pelvic fascia-sparing robot-assisted radical prostatectomy (PFS-RARP), a technique in which the endopelvic fascia is preserved by using a posterior approach to the prostate excision, was described in 2010 by Galfano et al., who demonstrated a faster return of urinary continence after surgery[8]. The development of PFS-RARP was driven by the need to further minimize the negative impacts of RP on urinary and sexual function, considering the role of the endopelvic fascia in these functions. Although PFS-RARP has been associated with improved functional outcomes, further study is required to determine if PFS-RARP confers long-term urinary outcomes benefit, or if oncologic outcomes differ compared to S-RARP. As a result of ongoing questions regarding oncologic efficacy, with some studies demonstrating an increase in the rate of positive surgical margins in the PFS-RARP approach, there continues to be disagreement in the field as to whether PFS-RARP can be employed as a favorable substitute for S-RARP[9]. In addition, there have been concerns regarding a steep learning curve associated with the PFS approach. Finally, a lack of randomized data raises questions about the reliability of data comparing PFS-RARP to more conventional techniques, with most studies comprising single-institutional and retrospective data[10].
The aim of this manuscript is to summarize the literature comparing functional and oncologic outcomes of PFS-RARP to S-RARP and outline the direction for future research comparing PFS-RARP to more conventional approaches.
PROCEDURAL BACKGROUND
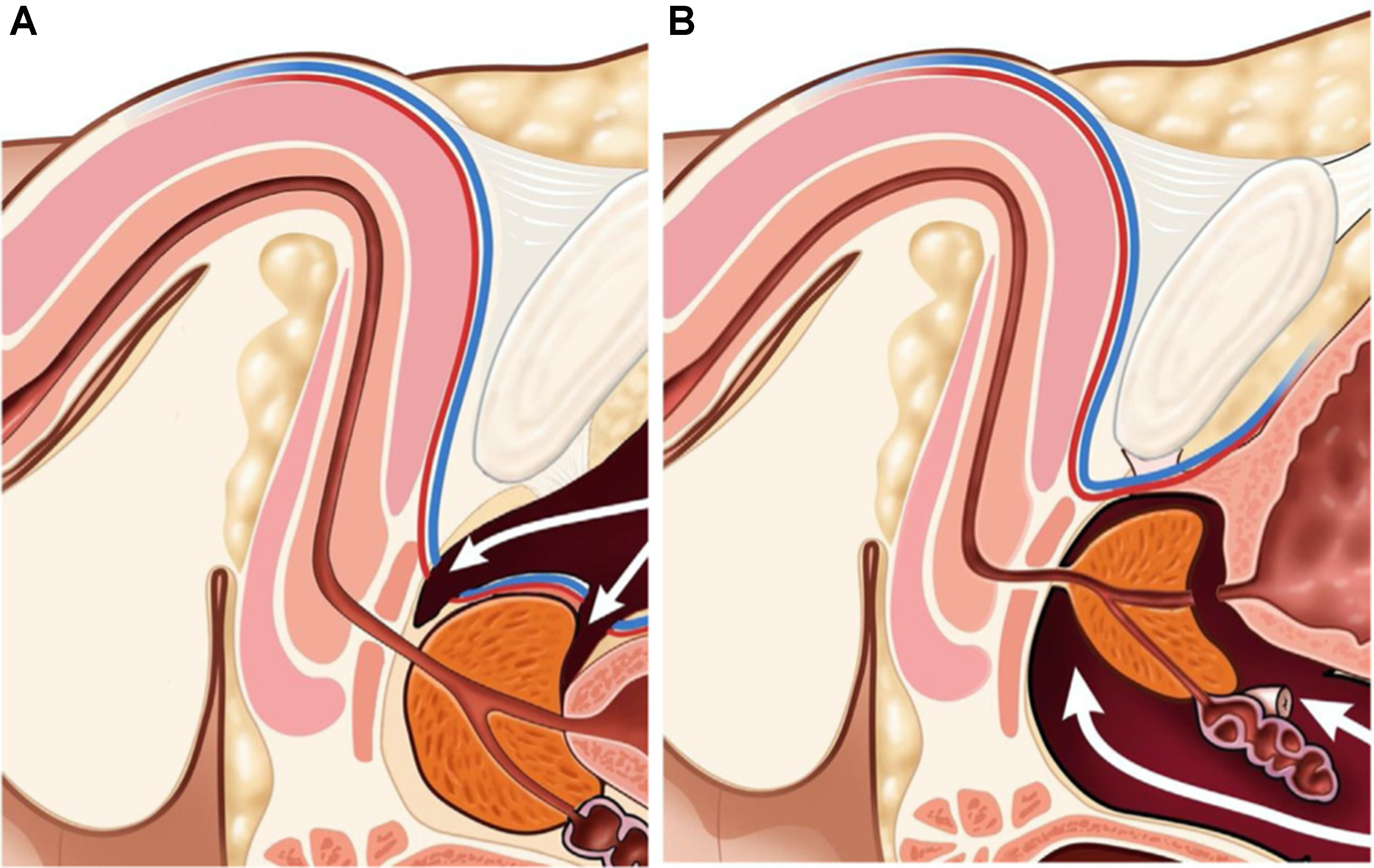
The PFS approach to the robot-assisted radical prostatectomy has been previously described[11]. S-RARP uses an anterior approach that involves transecting the anterior detrusor apron, which is a thin layer of muscle tissue that plays a role in pelvic floor support. As shown in Figure 1A, this disruption may contribute to postoperative complications related to a lack of pelvic floor support, including urinary incontinence. Additionally, the anterior approach in S-RARP requires transection of the puboprostatic ligaments and the dorsal venous complex (DVC) [Figure 1A], structures also thought to aid in pelvic floor support and urinary continence[11]. In contrast, PFS-RARP spares the endopelvic fascia, detrusor apron, puboprostatic ligaments, and the DVC[11] [Figure 1B], preserving these structures, which contribute to pelvic floor support and continence. Additionally, branches from the pudendal artery, supplying blood to the urethral sphincter and corpora cavernosum, may be encountered during ligation of the DVC in S-RARP[12]. The sparing of branches from the pudendal artery in PFS-RARP may lead to improved continence and erectile function, as supported by Droupy et al., whose cadaveric dissections show the crucial role of these arteries in supplying blood to the relevant tissues, particularly the corpora cavernosa[13]. Finally, though nerve-sparing S-RARP is often employed to preserve maximal sexual function, a cadaveric study has shown that approximately one-third of periprostatic nerves travel anterolaterally[14]. With PFS-RARP accessing the prostate posteriorly, it may also decrease injury to these anterolateral periprostatic nerves involved in sexual function[15].
Figure 1. Sagittal pelvic view during (A) S-RARP and (B) PFS-RARP. Disruption of the dorsal vascular complex at both the bladder neck and the prostate apex during S-RARP (A) contributes to worsened functional outcomes, such as urinary incontinence and potency. The dorsal vascular complex is left intact during PFS-RARP (B). S-RARP: Standard robot-assisted radical prostatectomy; PFS-RARP: pelvic fascia-sparing robotic-assisted radical prostatectomy.
OUTCOMES IN PFS-RARP VS. S-RARP
Oncologic outcomes
There is controversy in the literature regarding long-term oncologic outcomes of PFS-RARP vs. S-RARP[16]. One meta-analysis, including four RCTs and six prospective observational studies, demonstrated a statistically significant increase in PSMs in ≤ pT2 tumors for the PFS-RARP group vs. the S-RARP group [risk ratio (RR) 1.39; 95% confidence interval (CI) 1.01-1.91], and a non-significant increase in PSMs in ≥ pT3 tumors in the PFS-RARP group (RR 1.36; 95%CI 0.74-2.50). Seven of the included studies also reported biochemical recurrence (BCR) rates of disease. Two of those studies showed that BCR was significantly higher in the S-RARP group than in the PFS-RARP group, and the other five studies found no difference in BCR, all at a median follow-up of one year[16]. However, given the short follow-up of these studies, longer-term studies are needed. Another systematic review and meta-analysis demonstrated similar results. They found an overall increased likelihood of PSMs in the PFS-RARP group vs. the S-RARP group [odds ratio (OR) 1.71; 95%CI 1.12-2.60, P = 0.01]. When stratifying for pT stage, the difference trended toward more PSMs in pT2 tumors (OR 1.75; 95%CI 0.95-3.22, P = 0.07), but rates of PSMs were similar in ≥ pT3 tumors (OR 1.35; 95%CI 0.69-2.65, P = 0.37). Most of these PSMs were focal (< 3 mm), and the clinical significance of focal PSMs remains unclear[17]. Several studies have found that focal PSMs are not significantly associated with higher rates of BCR[18,19], while some experts argue that any margin positivity should impact what is done next, and NCCN guidelines even consider margin positivity an indication for adjuvant radiation[20].
This systematic review also reports that many of these PSMs in PFS-RARP were anterior to the prostate and occurred in cases of anterior primary tumors, concluding that the posterior approach of PFS-RARP is not advisable in the case of an anterior primary tumor[17]. Indeed, a large single-surgeon series found no differences in overall PSM rate between the PFS-RARP and S-RARP groups (35% vs. 25%, P = 0.067) but did find a significant difference between groups when stratifying for location of PSM, where the anterior PSM rate was 47% in the PFS-RARP group and 26% in the S-RARP group (P = 0.035)[21].
Additionally, a randomized study by Dalela et al. demonstrated that patients undergoing PFS-RARP had nearly double the rate of PSMs compared to S-RARP, though this difference was statistically insignificant (25% vs. 13%, P = 0.1). However, a relatively higher, though insignificant, proportion of T3 tumors were treated in the PFS-RARP group than the S-RARP group (45% vs. 23.3% T3 tumors, P = 0.4), and the sample size was only 60 patients in each arm, making it impossible to extrapolate these findings. On the other hand, the rate of BCR-free survival at one year was 91% in both groups (P = 0.5)[22]. Again, larger studies and longer follow-up are needed to determine the implications of these findings over time.
Some studies report no difference in short-term oncologic outcomes of PFS-RARP vs. S-RARP. Kadhim
To assess possible learning curve effects, Galfano et al. reviewed their first 200 PFS-RARP cases and compared outcomes between the initial 100 procedures and the subsequent 100. They found no differences in key outcomes (postoperative adverse events, continence, potency, and 1-year BCR) between the two groups with the exception of surgical margins (32% vs. 19%; P = 0.039)[8]. Other studies have suggested that there is no difference in outcomes for those new to the procedure. Olivero et al. used propensity score matching to compare experienced surgeons (ESs) to learning curve surgeons (LCSs) and found the PSM rate to be 25.4% and 19.9%, respectively (P = 0.13), where ESs had done greater than 100 procedures and LCSs were still on their first 100 procedures[26]. A single center in Hong Kong also used propensity score matching to assess any differences in outcomes during the learning curve period and found the positive surgical margin rate to be 29.2% in the PFS-RARP group compared to 54.1% in the S-RARP group (P = 0.142) during the first 24 cases[27], again demonstrating no differences in PSM rate due to a learning curve.
There is still conflicting evidence as to whether PFS-RARP is associated with worse long-term oncologic outcomes compared to S-RARP. It is unclear whether increased PSMs correlate to worse long-term oncologic outcomes, with all studies showing no difference in, or even decreased, BCR at one year postoperatively in PFS-RARP compared to S-RARP[11,22,25], and one study showing similar rates of BCR at four years, though without direct comparison[24]. Larger cohorts, randomized controlled trials, and longer follow-up are needed to determine the significance or reproducibility of increased PSMs in PFS-RARP, as well as to determine if there are confounding factors such as adjuvant chemotherapy, anterior primary tumors, or more aggressive tumors that influence long-term oncologic outcomes.
Perioperative outcomes
Studies comparing PFS-RARP to S-RARP have found no differences in perioperative outcomes such as postoperative complications, length of stay, and operative time[9,28,29]. Kowalczyk et al. reported a mean estimated blood loss (EBL) of 187 mL for those undergoing PFS-RARP as compared to 325 mL in S-RARP
Urinary continence
Several studies have demonstrated improvements in short-term urinary continence rates among men who undergo PFS-RARP compared to those undergoing conventional approaches. More recent evidence, including one meta-analysis, demonstrated improvements in long-term continence. However, some studies have shown loss of significant differences after several months of follow-up, and no difference at one year. While there is no explicit definition of short-term or long-term continence in the literature, most studies refer to around one year when speaking of short- or long-term.
An initial report of functional outcomes after PFS-RARP showed an average urinary continence rate of 91% at seven days after postoperative catheter removal[8]. Tahra et al. directly compared PFS-RARP to S-RARP and observed statistically significant differences in continence the first month after surgery (89.1% vs. 59.5%, P = 0.001), favoring PFS-RARP, where continence was defined as 0 pads[29]. Similarly, Dalela et al. found significant differences in continence using definitions of 0 pads/one security pad per day, 0 pads/day, and a 24-hour pad weight in grams at one week after catheter removal, all favoring PFS-RARP (P = 0.01, P = 0.001, and P = 0.002, respectively). Additionally, using pad weights, the authors noted increased continence at one and two weeks, and at one month postoperatively for the PFS-RARP group compared to the S-RARP group (P = 0.002, P = 0.004, P = 0.001, respectively)[22]. Time to continence differed significantly in the study by Egan et al., showing an average time to continence as defined by 0 pads of 59 days for PFS-RARP vs. 182 days for S-RARP (P < 0.001)[11].
Kadhim et al. looked at urinary continence at three months postoperatively, with one definition of continence being “pad-free status”, and another “social continence”, defined as ≤ 1 pad/24-hour period, with both yielding noteworthy results. Pad-free status was achieved in 72.9% of PFS-RARP patients at three months vs. 53.2% of S-RARP patients (P = 0.005), and social continence achieved in 88.1% vs. 77.2% (P = 0.056). After adjusting for confounding factors such as membranous urethral length, nerve-sparing procedures, and basal metabolic index, the multivariable logistic regression showed that PFS-RARP technique was independently associated with increased urinary continence at three months with an OR of 2.19 (95%CI 1.15-4.16, P = 0.017)[23].
While the time to return of urinary continence has consistently been shorter after PFS-RARP than S-RARP, one paper criticized using this as an outcome of interest, stating that a cancer-oriented surgery should not focus so much on only immediate differences in functional outcomes[31]. The critique notes that in one study, by one and three months postoperatively, significant differences in urinary function-related “bother” scores had disappeared[22]. This loss of long-term significance is also supported by Tahra et al., with data showing their early significant difference in continence was lost at one year postoperatively, when no difference in continence was observed between the two groups (93.5% vs. 90.5%, P = 0.6)[29]. This raises the question of long-term, clinically significant differences in continence between the two methods. However, it should be noted that these studies all used varying definitions of “continence” as a binary outcome rather than validated patient-reported quality of life questionnaires, which may better reflect overall urinary function and patient satisfaction. Long-term improvements in continence and bother following PFS-RARP were observed by Egan et al., and again in Dall et al., at a median of 12.3- and 26-month follow-up, respectively[11,21]. Using Expanded Prostate Cancer Index Composite for Clinical Practice (EPIC-CP) urinary incontinence symptom scores, Egan et al. found a significant difference in urinary incontinence between PFS-RARP and S-RARP at all time periods, including after 12 months [mean score 1.0 vs. 2.3 (higher score = worse symptoms), respectively, P = 0.033][11]. This is even more valuable, as studies have shown that the minimum clinically important difference for EPIC-CP urinary incontinence scores is 1.0[32]. Utilizing a conservative definition of achieving urinary continence (0-1 safety pad/day), there was still a significant difference in continence rates at 12 months (97.6% PFS-RARP vs. 81.4% S-RARP, P = 0.002). Finally, multivariate logistic regression analysis showed that PFS-RARP was independently associated with a decreased risk of incontinence at 12 months[11]. This reduction in EPIC-CP UI scores was re-demonstrated by Dall et al., even at 24 months (EPIC-CP UI score 1.2 in PFS-RARP vs. 2.2 in S-RARP, P = 0.001). In multivariate analysis, PFS-RARP was independently associated with improved EPIC-CP UI and total quality of life scores at 18 months, and while not significant, still trended toward improvement with PFS-RARP at 24 months[21]. This is the first study to show that improvements in continence and, more importantly, quality of life, persist to 24 months after RS-RARP compared directly to S-RARP.
A 2020 meta-analysis also found long-term benefits, defining continence as the use of 0-1 safety pad/day. It reported a higher likelihood of continence 12 months after surgery for the PFS-RARP group (OR 7.29, 95%CI 1.89-28.13, P = 0.004)[17]. Finally, a more recent meta-analysis also found increased rates of continence at 12 months in the PFS-RARP groups (OR 4.37; 95%CI 1.97-9.73)[25]. Future research, particularly studies with longer follow-up and more meta-analyses, should continue to explore long-term differences in continence to form a consensus on the significance of this outcome. Additionally, the use of different definitions of continence across studies, such as 0 pads, 0-1 safety pad/day, or “pad-free” status, complicates the comparison of urinary continence outcomes between studies. In future research, there is a need for a standardized definition to accurately assess the long-term benefits of PFS-RARP on continence. Finally, in addition to the objective measures of continence, patient-reported experiences regarding how improved continence affects their daily life, such as participation in social activities or sleep quality, should also be considered to fully understand the long-term significance of continence differences between PFS-RARP and S-RARP.
Sexual function
Erectile dysfunction at 12 months after S-RARP has been estimated to have a range of 19%-45% as reported in one meta-analysis[33] and remains a major quality of life concern.
Several studies have postulated that preservation of the same structures spared in PFS-RARP, specifically the pudendal arteries, endopelvic fascia, and DVC, may lead to improved potency and sexual function[9]. A recent study reports 86.7% of potency recovery at 12 months following a modified RARP technique, in which the aforementioned structures are left intact, as compared to the estimated 70% return to potency at 12 months after S-RARP in the literature[15]. However, the theoretical improvements in sexual function attributed to improved pelvic anatomy preservation have not been reliably demonstrated in comparative studies.
Overall, the evidence regarding the impact of PFS-RARP on potency is inconclusive. Some studies suggest a potential increase in potency, while others find no significant difference. For instance, Egan et al. reported no significant differences in sexual function at 3, 6, 9, or 12 months as measured by EPIC-CP sexual function scores[11]. Menon et al. assessed potency based on the ability to achieve an erection sufficient for penetrative intercourse at least half the time. Similar to Egan et al., their RCT found no significant difference in potency outcomes. While statistical significance has not been shown, there appears to be an insignificant increase in potency following PFS-RARP in some studies. In Egan et al.’s study, at 3 months, 43.7% of patients in the PFS-RARP group vs. 36.7% in the S-RARP group had potency, and at 12 months, 86.5% vs. 69.2% had potency (P = 0.5)[34]. Additionally, at 3 months following surgery, fewer men used phosphodiesterase-5 inhibitors (P = 0.30) and/or intracavernosal injections (P = 0.40) in the PFS-RARP group. However, Umari et al., using the IIEF-5 score, report no significant difference in potency for their cohort of nearly 500 patients either immediately after surgery or at 1, 3, 6, 9, and 12 months[35].
In theory, a surgical approach that avoids the transection of the endopelvic fascia and the DVC should maintain erectile function; however, this has not been supported in comparative studies nor in meta-analyses[16,25]. A serious limitation in the assessment of PFS-RARP as it relates to postoperative potency rates is the current lack of standardization in assessing potency and erectile function. To address this lack of standardization, future studies should consider using a combination of validated questionnaires, such as the IIEF-5 and EPIC-CP sexual function scores, along with objective measures like nocturnal penile tumescence monitoring. Finally, with the preference for performing nerve-sparing S-RARP when possible, it is also necessary to direct future research to assess any differences between PFS-RARP and S-RARP after stratifying for no, unilateral, or bilateral nerve-sparing S-RARP procedures.
Additional postoperative sequelae of radical prostatectomy
Penile shortening
In addition to urinary continence and sexual function, there are other consequences of radical prostatectomy that should be considered when comparing the outcomes of PFS-RARP and S-RARP. Penile shortening is a known but less mentioned consequence of RP, with Gontero et al. describing a significant degree of penile shortening one year after RP[36]. It has been postulated that preservation of the natural arterial and venous supply to the penis in PFS-RARP could result in less penile shortening than following a procedure in which these structures are disrupted[37]. The study by Kowalczyk et al. is the only one thus far directly comparing the degree of penile shortening between PFS-RARP and S-RARP. The prospective observational study found that PFS-RARP was associated with significantly fewer patients reporting subjective penile shortening than S-RARP (41.7% vs. 66.7%, P = 0.012). This association was still significant in adjusted analyses. However, the study measured penile shortening based on the subjective patient descriptions [i.e., “Is your penile length subjectively shorter compared to before prostatectomy (yes or no)?”] and is a source of bias in the study[10].
Penile shortening is associated with decreased self-esteem and quality of life. One study found a significantly higher prevalence of low-to-moderate self-assessed quality of life and low-to-moderate self-reported self-esteem among prostate cancer survivors with perceived penile shortening postoperatively vs. those without[37]. This is also a potentially under-studied side effect of RP, as none of the other recent studies comparing PFS-RARP to S-RARP have included penile shortening as an endpoint of interest. Further research is warranted to confirm the results of this study and evaluate other factors that may be related to this bothersome outcome. In addition, to overcome the limitation of short follow-up, future studies should conduct long-term follow-up of at least 3-5 years and use more objective measures of penile length, such as ultrasound-based measurements, which could reduce the bias associated with subjective patient reports.
Peyronie’s disease
Peyronie’s disease (PD) is another adverse outcome that has been shown to be related to RP, with one study finding an incidence of PD of 15.9% within three years after RP, higher than even the highest estimated incidence of 8.9% in the general population[38]. However, there has been little literature on the relationship between PD and different RP techniques. Kowalczyk et al. also used PD as an endpoint in patients undergoing PFS-RARP vs. S-RARP. The study found significantly less PD in those undergoing PFS-RARP than those undergoing S-RARP, with 0 cases of PD reported at a median follow-up of 14 months after PFS-RARP and 5 cases (8.7%) reported at a median follow-up of 55 months after S-RARP. PD was self-reported on a questionnaire asking, “Have you noticed any new penile curvature or deformity in a flaccid or erect state following prostatectomy?” and validated through blinded clinical examination with 100% sensitivity and 99.4% specificity[10]. The short follow-up time for the PFS-RARP group, as compared to S-RARP, may contribute to bias. Only 7.6% of participants had developed PD by one year after RP in the study by Tal
Inguinal hernia
Finally, a third outcome measured in our most recent study was inguinal hernia after PFS-RARP or S-RARP. Inguinal hernia is a known complication of RRP and S-RARP but has been largely ignored as an outcome in comparative studies[39]. One meta-analysis demonstrated a significantly increased incidence of inguinal hernia after RARP as compared to a control group undergoing transurethral resection of the prostate (4.2% incidence vs. 0.5%, P < 0.001), highlighting inguinal hernia as a major adverse outcome of RP[40].
Kowalczyk et al. found a significantly decreased number of inguinal hernias postoperatively in the PFS-RARP group as compared to the S-RARP group (0% vs. 12.3%, P = 0.006). Again, however, the median follow-up time for PFS-RARP was 14 months[10], and this relatively short follow-up time could bias the results, as inguinal hernias may develop over a longer period of time. Indeed, inguinal hernias typically develop in the first two to three years after RP[40]. A longer follow-up period is essential to evaluating the true incidence following PFS-RARP.
ALTERNATIVES TO RS-RARP
In 2020, Wagaskar et al. published initial single-surgeon outcomes of their modified RARP called the “hood technique”, an anterior approach that spares supporting anterior fascia via dissection between the detrusor apron and the anterior layer of the prostate. The preserved tissue, containing the detrusor apron, arcus tendineus, puboprostatic ligament, anterior vessels, and part of the detrusor muscle, has the appearance of a “hood” after removal of the prostate, hence the name. Wagaskar et al. included 300 patients undergoing RARP using the hood technique in their analysis and examined outcomes of oncologic efficacy and return to continence. Their positive surgical margin rate was 6% total (2.3% in pT2 tumors and 3.7% in pT3 tumors), lower than average reported PFS-RARP positive surgical margin rates, but they did not specify whether these PSMs were focal or non-focal. Importantly, unlike prior PFS-RARP studies, this analysis excluded patients with anterior primary tumors[41]. The complication rate was similar to the complication rates of PFS-RARP (9.7%). Continence, defined as 0 pads, was 21% at the first week after catheter removal and 36% by the second week. Continence continued to improve to 88% at 6 weeks and 91% by 12 weeks, showing an early return to continence. By the end of the 12-month follow-up period, only 5% were incontinent, with the majority using only one pad per day[41].
The series did not directly compare this modified technique to S-RARP. It is also limited by the technique being performed by one ES, raising similar questions about its reproducibility among trainees or those less experienced with RARP. Additionally, sexual outcomes are unknown, although future research is underway to examine any effects on postoperative sexual function[42].
Nevertheless, the hood technique provides another methodology for sparing anterior fascial support during RARP and again shows impressive continence rates compared with S-RARP, re-emphasizing the importance of these structures in maintaining urinary continence. While the hood technique shows promising continence rates, ultimately, future prospective trials by other surgeons as well as randomized controlled trials will be necessary to validate reproducible outcomes and directly compare the technique to PFS-RARP and S-RARP.
DIRECTIONS FOR FUTURE RESEARCH
Overall, the main limitation in assessing significant differences between PFS-RARP and S-RARP is the short follow-up period for the PFS-RARP group in most studies. Future studies should continue to explore PSMs and BCR for a longer follow-up period to further determine if there is a significant difference in PSMs, and whether such a difference has clinically relevant implications for the recurrence of disease. Longer follow-up is also important to determine statistically significant differences in potency, penile shortening, PD, and postoperative inguinal hernias. Finally, while earlier return to continence is a widely accepted benefit of PFS-RARP, longer follow-up and RCTs with greater numbers of participants are important to determine if there is a long-term benefit in continence, as several studies have begun to suggest. These persistent questions regarding oncologic efficacy, learning curve, and secondary outcomes of PFS-RARP have prompted the recent allotment of support from the National Institutes of Health toward a multi-center, randomized controlled trial evaluating outcomes after S-RARP and PFS-RARP: the Clinical TrIAL of Approaches to Prostate cAncer suRgery (PARTIAL) trial hopes to recruit 600 participants and follow them for four years to further parse these differences[43]. Other future RCTs should also ensure a large and diverse patient population, with proper stratification based on factors such as tumor stage, Gleason score, and patient age, and strive to maintain a follow-up period of 3-5 years to accurately assess long-term outcomes.
CONCLUSION
PFS-RARP has superior urinary function outcomes compared with standard radical prostatectomy, particularly in the early postoperative period. While some data suggest higher positive surgical margin rates, longer follow-up is needed to determine if more clinically relevant oncologic outcomes differ between the two procedures. Current and future studies should be multi-institutional and collaborative in order to determine if PFS-RARP should supplant RARP as the gold standard for the treatment of localized prostate cancer.
DECLARATIONS
Acknowledgments
Medical illustrations were done by David Klemm, Georgetown University Medical Center, Washington, DC, USA.
Authors’contributions
Drafting of the manuscript: Kodres-O’Brien S, Dall C
Critical revision of the manuscript: Kodres-O’Brien S, Dall C, Sholklapper T, Kowalczyk K
Administrative, technical, and material support: Kowalczyk K
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. AUANews. Westerman ME. Robotic prostatectomy: a game-changer in prostate cancer treatment. 2024. Available from: https://auanews.net/issues/articles/2024/february-2024/robotics-robotic-prostatectomy-a-game-changer-in-prostate-cancer-treatment. [Last accessed on 11 Jul 2025].
2. Lane A, Metcalfe C, Young GJ, et al; ProtecT Study group. Patient-reported outcomes in the ProtecT randomized trial of clinically localized prostate cancer treatments: study design, and baseline urinary, bowel and sexual function and quality of life. BJU Int. 2016;118:869-79.
3. Hoffman KE, Penson DF, Zhao Z, et al. Patient-reported outcomes through 5 years for active surveillance, surgery, brachytherapy, or external beam radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA. 2020;323:149-63.
4. Shi Z. Laparoscopic vs. open surgery: a comparative analysis of wound infection rates and recovery outcomes. Int Wound J. 2024;21:e14474.
5. De Carlo F, Celestino F, Verri C, Masedu F, Liberati E, Di Stasi SM. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: surgical, oncological, and functional outcomes: a systematic review. Urol Int. 2014;93:373-83.
6. Borregales LD, Berg WT, Tal O, et al. 'Trifecta' after radical prostatectomy: is there a standard definition? BJU Int. 2013;112:60-7.
7. Ficarra V, Novara G, Rosen RC, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2012;62:405-17.
8. Galfano A, Di Trapani D, Sozzi F, et al. Beyond the learning curve of the Retzius-sparing approach for robot-assisted laparoscopic radical prostatectomy: oncologic and functional results of the first 200 patients with ≥ 1 year of follow-up. Eur Urol. 2013;64:974-80.
9. Davis M, Egan J, Marhamati S, Galfano A, Kowalczyk KJ. Retzius-sparing robot-assisted robotic prostatectomy: past, present, and future. Urol Clin North Am. 2021;48:11-23.
10. Kowalczyk KJ, Davis M, O’Neill J, et al. Impact of Retzius-sparing versus standard robotic-assisted radical prostatectomy on penile shortening, Peyronie’s disease, and inguinal hernia sequelae. Eur Urol Open Sci. 2020;22:17-22.
11. Egan J, Marhamati S, Carvalho FLF, et al. Retzius-sparing robot-assisted radical prostatectomy leads to durable improvement in urinary function and quality of life versus standard robot-assisted radical prostatectomy without compromise on oncologic efficacy: single-surgeon series and step-by-step guide. Eur Urol. 2021;79:839-57.
12. Mulhall JP, Secin FP, Guillonneau B. Artery sparing radical prostatectomy - myth or reality? J Urol. 2008;179:827-31.
13. Droupy S, Benoît G, Giuliano F, Jardin A. Penile arteries in humans. Origin - distribution - variations. Surg Radiol Anat. 1997;19:161-7.
14. Alsaid B, Bessede T, Diallo D, et al. Division of autonomic nerves within the neurovascular bundles distally into corpora cavernosa and corpus spongiosum components: immunohistochemical confirmation with three-dimensional reconstruction. Eur Urol. 2011;59:902-9.
15. de Carvalho PA, Barbosa JABA, Guglielmetti GB, et al. Retrograde release of the neurovascular bundle with preservation of dorsal venous complex during robot-assisted radical prostatectomy: optimizing functional outcomes. Eur Urol. 2020;77:628-35.
16. Barakat B, Othman H, Gauger U, Wolff I, Hadaschik B, Rehme C. Retzius sparing radical prostatectomy versus robot-assisted radical prostatectomy: which technique is more beneficial for prostate cancer patients (MASTER study)? A systematic review and meta-analysis. Eur Urol Focus. 2022;8:1060-71.
17. Checcucci E, Veccia A, Fiori C, et al. Retzius-sparing robot-assisted radical prostatectomy vs the standard approach: a systematic review and analysis of comparative outcomes. BJU Int. 2020;125:8-16.
18. Porcaro AB, Tafuri A, Sebben M, et al. Linear extent of positive surgical margin impacts biochemical recurrence after robot-assisted radical prostatectomy in a high-volume center. J Robot Surg. 2020;14:663-75.
19. Lee S, Kim KB, Jo JK, et al. Prognostic value of focal positive surgical margins after radical prostatectomy. Clin Genitourin Cancer. 2016;14:e313-9.
20. Grypari IM, Zolota V, Tzelepi V. Radical or not-so-radical prostatectomy: do surgical margins matter? Cancers. 2021;14:13.
21. Dall CP, Mason JB, Choudhury E, et al. Long-term outcomes of pelvic-fascia sparing robotic-assisted radical prostatectomy versus standard technique: superior urinary function and quality of life without compromising oncologic efficacy in a single-surgeon series. Urol Oncol. 2024;42:67.e17-24.
22. Dalela D, Jeong W, Prasad MA, et al. A pragmatic randomized controlled trial examining the impact of the Retzius-sparing approach on early urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2017;72:677-85.
23. Kadhim H, Ang KM, Tan WS, et al. Retzius-sparing technique independently predicts early recovery of urinary continence after robot-assisted radical prostatectomy. J Robot Surg. 2022;16:1419-26.
24. Dell’oglio P, Tappero S, Longoni M, et al. Retzius-sparing robot-assisted radical prostatectomy in high-risk prostate cancer patients: results from a large single institution series. Eur Urol Open Sci. 2022;38:69-78.
25. O’Connor-Cordova MA, Macías AGO, Sancen-Herrera JP, et al. Surgical and functional outcomes of Retzius-sparing robotic-assisted radical prostatectomy versus conventional robotic-assisted radical prostatectomy in patients with biopsy-confirmed prostate cancer. Are outcomes worth it? Systematic review and meta-analysis. Prostate. 2023;83:1395-414.
26. Olivero A, Galfano A, Piccinelli M, et al. Retzius-sparing robotic radical prostatectomy for surgeons in the learning curve: a propensity score-matching analysis. Eur Urol Focus. 2021;7:772-8.
27. Yee CH, Liu AQ, Chiu PKF, Teoh JYC, Hou SSM, Ng CF. A propensity score-matching study on Retzius-sparing robotic-assisted radical prostatectomy: evidence of continence advantage on the early learning curve. Asian J Surg. 2022;45:1403-7.
28. Wong D, Rincon J, Henning G, Smith Z, Kim E. Retzius sparing prostatectomy effect on symptomatic lymphocele rates. Urology. 2021;149:129-32.
29. Tahra A, Sen U, Sobay R, İnkaya A, Kucuk E, Boylu U. Comparison of Retzius-sparing versus standard robot-assisted radical prostatectomy for prostate cancer. Actas Urol Esp. 2022;46:293-300.
30. Deng W, Jiang H, Liu X, et al. Transvesical Retzius-sparing versus standard robot-assisted radical prostatectomy: a retrospective propensity score-adjusted analysis. Front Oncol. 2021;11:687010.
31. Stonier T, Simson N, Davis J, Challacombe B. Retzius-sparing robot-assisted radical prostatectomy (RS-RARP) vs standard RARP: it’s time for critical appraisal. BJU Int. 2019;123:5-7.
32. Chipman JJ, Sanda MG, Dunn RL, et al; PROST-QA Consortium. Measuring and predicting prostate cancer related quality of life changes using EPIC for clinical practice. J Urol. 2014;191:638-45.
33. Ficarra V, Novara G, Ahlering TE, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol. 2012;62:418-30.
34. Menon M, Dalela D, Jamil M, et al. Functional recovery, oncologic outcomes and postoperative complications after robot-assisted radical prostatectomy: an evidence-based analysis comparing the Retzius sparing and standard approaches. J Urol. 2018;199:1210-7.
35. Umari P, Eden C, Cahill D, Rizzo M, Eden D, Sooriakumaran P. Retzius-sparing versus standard robot-assisted radical prostatectomy: a comparative prospective study of nearly 500 patients. J Urol. 2021;205:780-90.
36. Gontero P, Galzerano M, Bartoletti R, et al. New insights into the pathogenesis of penile shortening after radical prostatectomy and the role of postoperative sexual function. J Urol. 2007;178:602-7.
37. Carlsson S, Nilsson AE, Johansson E, Nyberg T, Akre O, Steineck G. Self-perceived penile shortening after radical prostatectomy. Int J Impot Res. 2012;24:179-84.
38. Tal R, Heck M, Teloken P, Siegrist T, Nelson CJ, Mulhall JP. Peyronie’s disease following radical prostatectomy: incidence and predictors. J Sex Med. 2010;7:1254-61.
39. Würnschimmel C, Graefen M. Orphaned side-effects after robot-assisted radical prostatectomy: is the Retzius-sparing approach superior to the standard approach or are the data just not mature enough? Eur Urol Open Sci. 2021;23:34-5.
40. Alder R, Zetner D, Rosenberg J. Incidence of inguinal hernia after radical prostatectomy: a systematic review and meta-analysis. J Urol. 2020;203:265-74.
41. Wagaskar VG, Mittal A, Sobotka S, et al. Hood technique for robotic radical prostatectomy-preserving periurethral anatomical structures in the space of Retzius and sparing the pouch of douglas, enabling early return of continence without compromising surgical margin rates. Eur Urol. 2021;80:213-21.
42. Mount Sinai. Hood technique enables early return to continence following RARP. 2020. Available from: https://reports.mountsinai.org/article/hood-technique-enables-early-return-to-continence-following-rarp. [Last accessed on 11 Jul 2025].
43. ClinicalTrials.gov. Clinical trial of approaches to prostate cancer surgery (PARTIAL). 2025. Available from: https://www.clinicaltrials.gov/ct2/show/NCT05155501. [Last accessed on 11 Jul 2025].
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data



















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].