Artificial intelligence in interventional cardiology: current applications and future clinical integration
Abstract
Artificial intelligence (AI) has been revolutionizing invasive cardiology in recent years, with respect to diagnostic accuracy, procedural success, and long-term patient outcomes. Advanced machine learning (ML) and deep learning algorithms facilitate automated image analysis, risk stratification, and personalized intervention planning, paving the way for precision medicine. AI-based technologies, such as coronary computed tomography angiography, intravascular ultrasound, and optical coherence tomography, enable precise plaque definition and quantification that goes beyond classical subjective methods. AI-based quantitative computed tomography and radiomics-based approaches have demonstrated strong correlations with invasive standards such as NIRS-IVUS, effectively identifying lipid-rich plaques and predicting acute coronary events. AI is also refining risk stratification models, significantly improving predictive capabilities compared to traditional methods, thus enabling personalized therapeutic interventions in real time. In interventional cardiology, the integration of real-time AI with fluoroscopy significantly improves procedural decision making while reducing procedure time, radiation exposure, and operator variability. Additionally, AI-assisted predictive analytics facilitate comprehensive risk assessment, optimizing treatment strategies by accurately identifying patients at the highest risk of major adverse cardiovascular events. ML algorithms improve image analysis by automating plaque characterization, thus facilitating clinical decision making and procedural optimization. In the future, AI-based applications, such as AI-guided catheter navigation, could further transform PCI, opening new possibilities for innovation and optimization. Despite these advances, challenges remain regarding data standardization, algorithmic interpretability, regulatory compliance, and ethical concerns about data privacy and potential bias. This review will explore the risks and potential benefits of this unprecedented evolution.
Keywords
INTRODUCTION
Cardiovascular disease (CVD) remains one of the leading causes of mortality worldwide, with atherosclerosis acting as the primary pathological driver of acute myocardial infarction (MI) and stroke[1]. Over the years, interventional cardiology has primarily focused on the severity of stenosis and its implications for cardiovascular events. However, recent decades have witnessed a paradigm shift from a purely angiographic assessment toward a more comprehensive approach that incorporates functional evaluation, plaque composition, and morphological features to better determine plaque vulnerability and thrombotic risk[2,3]. Advancements in coronary imaging modalities, including coronary computed tomography angiography (CCTA), intravascular ultrasound (IVUS), and optical coherence tomography (OCT), have significantly improved the characterization of atherosclerotic plaque. Nevertheless, despite these technological innovations, operator-related variability remains a critical limitation, contributing to discrepancies in long-term prognostic assessments among patients[4].
In this context, artificial intelligence (AI) is emerging as a transformative tool capable of enhancing automation, standardization, and diagnostic precision. AI-based methodologies are reshaping cardiovascular risk prediction, not only by refining imaging analysis but also by integrating multimodal data sources to improve patient stratification. AI-driven models have consistently demonstrated superior performance compared to conventional cardiovascular risk scores, enabling the automated detection of ASCVD risk markers, including coronary artery calcium (CAC), across a variety of imaging modalities such as ECG-gated CT scans, chest X-rays, and non-gated chest CT scans[5]. Additionally, machine learning (ML) and deep learning (DL) algorithms facilitate automated segmentation, quantitative plaque assessment, and more precise risk prediction, ultimately refining traditional diagnostic workflows[6]. Among emerging AI-powered imaging tools, quantitative computed tomography (AI-QCT) has been shown to exhibit a strong correlation with IVUS-derived lipid quantification, further supporting its role in the noninvasive characterization of coronary plaques and cardiovascular risk assessment[7]. The ability of AI-QCT to assess lipid-rich plaques with high accuracy underscores its potential in the early identification of high-risk lesions, complementing intravascular imaging techniques. Furthermore, AI-guided fractional flow reserve CT (FFR-CT) integrates computational fluid dynamics (CFD) models to provide noninvasive hemodynamic assessments, enhancing ischemia detection and guiding clinical decision making in interventional cardiology. AI applications are also proving instrumental in real-time lesion characterization, procedural guidance, and stent optimization, thereby improving accuracy while minimizing procedural duration and radiation exposure[6]. AI-powered cardiovascular imaging analysis has demonstrated the capacity to refine interventional strategies, reduce operator dependency, and improve diagnostic reliability. Moreover, by integrating clinical data with advanced imaging techniques, AI-based systems can contribute to improved prognostic assessments and facilitate tailored therapeutic strategies for high-risk patients[8].
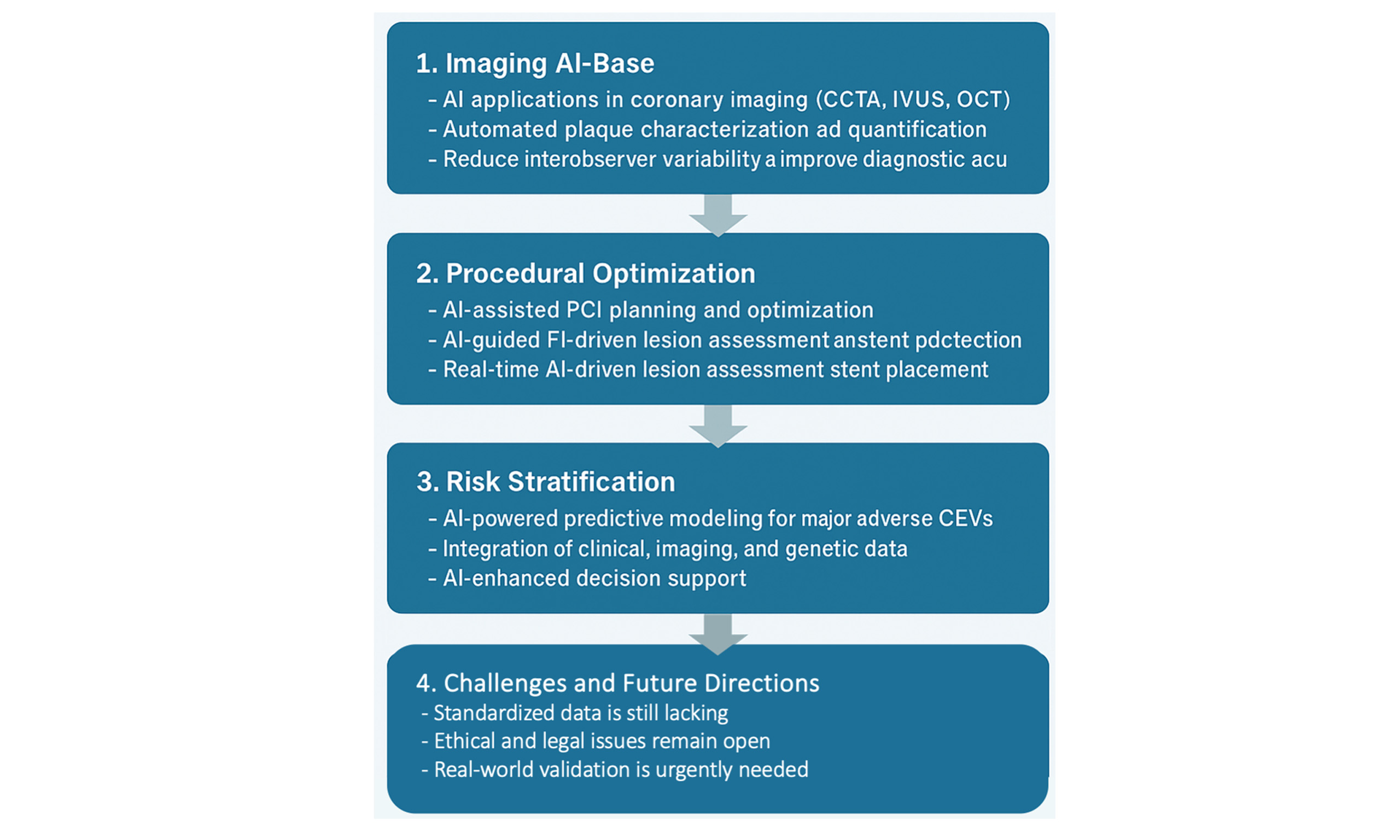
This review aims to critically examine how artificial intelligence is reshaping interventional cardiology. We not only provide an updated overview of current AI applications, but also offer a critical interpretation of emerging technologies, highlight implementation challenges, and reflect on future directions for clinical integration.
Figure 1 provides an overview of AI’s clinical applications in interventional cardiology, covering imaging, procedural guidance, and risk assessment.
Figure 1. Schematic representation of AI applications in interventional cardiology, categorized into imaging, diagnosis, interventional procedures, automation, and prognosis. CCTA: Coronary computed tomography angiography; IVUS: intravascular ultrasound; OCT: optical coherence tomography; AI-QCT: artificial intelligence-based quantitative computed tomography; ML: machine learning; AI-QCA: artificial intelligence-based quantitative coronary angiography; FFR-CT: fractional flow reserve derived from computed tomography; PCI: percutaneous coronary intervention; MACE: major adverse cardiovascular events.
AI IN CCTA FOR PLAQUE CHARACTERIZATION
AI has the potential to substantially augment the diagnostic and prognostic utility of CCTA by enhancing plaque characterization, refining stenosis quantification, and enabling a more granular approach to risk stratification[9]. Traditional CCTA interpretation, being inherently subjective, remains susceptible to interobserver variability, quality-dependent biases, and diagnostic inconsistencies. In contrast, AI-driven methodologies - particularly ML and DL algorithms - facilitate automated, high-throughput, and reproducible analyses, fostering a more standardized, objective, and precise assessment of plaque burden, composition, and morphological complexity[10].
Automated plaque characterization and quantification
Recent studies have demonstrated how AI improves plaque segmentation and classification, distinguishing calcified, non-calcified, and lipid-rich plaques with high sensitivity and specificity[11]. A study by
Advancements in AI-driven coronary plaque analysis have expanded the ability to quantify high-risk plaque features and total atherosclerotic burden. The DECODE study showed that AI-based plaque quantification altered clinical decision making in 66% of patients, demonstrating its potential impact on patient management strategies[12]. Furthermore, AI models allow real-time automated assessment of non-calcified plaques, which are often undetected by conventional CCTA-based methods, improving early risk stratification[13]. These capabilities, combined with AI-powered radiomics, enable more precise phenotyping of plaque instability, allowing tailored interventions for high-risk individuals[14].
In the CREDENCE[15] trial, AI-QCT showed higher sensitivity (95% vs. 74%) and specificity (63% vs. 43%) compared to myocardial perfusion imaging (MPI) for the detection of obstructive CAD. Furthermore, a post hoc analysis of the CONSERVE trial[16] applied AI-QCT retrospectively to 747 stable patients, showing that its use could have reduced unnecessary invasive coronary angiography (ICA) by 87%-95%, while lowering costs by 26%-34%, without compromising 1-year safety outcomes, as no cardiovascular deaths or myocardial infarctions occurred in 78% of patients with < 50% stenosis.
In addition to its diagnostic precision, AI-QCT offers a standardized and reproducible approach to total plaque burden assessment, addressing the inherent limitations of qualitative methods such as the segment involvement score (SIS), coronary artery calcium score (CACS), visual assessment, and CAD-RADS stenosis categories. In a comparative study, Khan et al. reported that AI-QCT demonstrated high concordance with SIS (93%, k = 0.87) and moderate agreement with CAD-RADS stenosis categories (73%), CACS (66%), and visual plaque assessment (64%)[17]. Notably, AI-QCT identified small, non-calcified plaques that were often undetected by traditional methods, underscoring its potential to enhance the precision of atherosclerosis quantification in routine clinical practice.
Beyond diagnostic improvements, AI-QCT also enhances risk stratification and clinical decision making. By integrating lesion morphology, volume, and hemodynamic relevance, AI-derived plaque burden has demonstrated stronger correlations with major adverse cardiovascular events (MACE) compared to CAD-RADS, offering a more comprehensive framework for long-term risk assessment. Furthermore, its ability to standardize image interpretation across different centers reduces inter-reader variability and improves reproducibility, reinforcing its role in optimizing patient management strategies[12,13].
AI-driven fractional flow reserve (FFR-CT) for functional assessment
AI could significantly enhance the assessment of coronary stenoses through AI-guided fractional FFR-CT. Conventional visual classification of stenosis often correlates poorly with ischemic burden, leading to misclassification of lesion severity[18]. Integrating computational fluid dynamics (CFD) and artificial intelligence, these models enable a noninvasive, physiology-driven assessment that refines PCI decision making and reduces unnecessary interventions. A meta-analysis of over 6,000 lesions confirmed that machine learning-based FFR-CT achieves diagnostic accuracy and specificity comparable to CFD-derived methods, with no significant difference between the two, despite a modest reduction in sensitivity[19].
Recent advancements in ML-guided FFR-CT algorithms have achieved area under the curve (AUC) values as high as 0.97, outperforming traditional CCTA-derived metrics in predicting ischemia[20]. In a comparative study, Chiou et al. found that an AI-based quantitative CT algorithm outperformed CT-FFR and physician visual assessment in predicting functionally significant coronary lesions, achieving an AUC of 0.91 vs. 0.76 for CT-FFR and 0.62 for CAD-RADS. Notably, AI maintained superior specificity even in patients with high calcified burden, where CT-FFR performance declines[21].
The clinical utility of AI-assisted FFR-CT has been supported by evidence showing that it can reduce unnecessary invasive coronary angiography (ICA) by up to 49% while maintaining high diagnostic accuracy[15]. A large-scale analysis from the ADVANCE registry demonstrated that AI-enabled quantitative coronary plaque analysis (AI-QCPA) significantly correlates with one-year adverse cardiac events and late revascularization, reinforcing its predictive value beyond luminal stenosis alone[22]. The reproducibility of FFR-CT measurements was assessed in a repeatability study using different AI-based software platforms, revealing that image quality, heart rate variability, and vessel shifting length significantly influence measurement accuracy[23].
Recent comparisons of AI-based FFR prediction models and CFD-derived FFRCT have shown promising results in patients with intermediate-grade stenosis. Peters et al. demonstrated that an AI DL model for FFR prediction achieved 91% sensitivity, 82% specificity, and an overall diagnostic accuracy of 85%, performing comparably to conventional CFD-based FFRCT. The AI model provided faster analysis while maintaining a high negative predictive value (96%)[18]. A further recent study introduced a fully automated on-site AI-based CT-FFR technology, achieving a 99.8% technical success rate and 82% diagnostic accuracy, with a significant reduction in computation time to under four minutes, making it a more efficient alternative for functional assessment and risk stratification[24]. This clinical relevance has been recently supported by the TARGET trial, a multicenter randomized study demonstrating that on-site AI-based CT-FFR significantly reduced unnecessary invasive coronary angiographies in patients with stable CAD, though without a significant impact on symptoms, major adverse cardiovascular events, or quality of life[25].
AI in risk stratification and prognostic modeling
The role of AI in risk stratification extends beyond static plaque assessment alone. Emerging AI-based predictive models integrate clinical, genetic, and imaging data, facilitating real-time risk assessment and longitudinal plaque monitoring[17]. AI-based CCTA biomarkers, such as perivascular fat attenuation index (FAI), plaque vulnerability scores, and hemodynamic stress markers, have emerged as robust predictors of cardiovascular events. Recent findings suggest that AI-driven CCTA biomarker extraction combined with computational risk models can significantly improve long-term cardiovascular risk prediction compared to traditional scoring systems[26].
The ORFAN study demonstrated that AI-driven integration of perivascular fat attenuation index (FAI) scores significantly improves risk stratification, even in patients without obstructive CAD. FAI, a CCTA-derived marker of coronary inflammation, independently predicts cardiac mortality and MACE, refining risk assessment beyond traditional models. The validated AI-Risk algorithm, combining FAI, plaque burden, and clinical variables, outperforms conventional stratification, supporting its integration into clinical workflows[27].
Moreover, as demonstrated by recent findings, AI-driven analysis has refined risk stratification by incorporating fat-derived biomarkers that capture vascular inflammation and metabolic activity. Beyond traditional plaque assessment, AI-enhanced quantification of pericoronary adipose tissue (PCAT) attenuation has shown promise in identifying subclinical coronary inflammation, which is associated with plaque instability and adverse cardiovascular outcomes[28]. Additionally, automated segmentation of epicardial adipose tissue (EAT) has revealed correlations with metabolic risk factors, suggesting its potential role in refining cardiovascular risk models. These advancements highlight the evolving role of AI in integrating multi-dimensional imaging biomarkers to improve patient-specific prognostic assessment[29].
A schematic representation of an AI-enhanced diagnostic workflow is provided in Figure 2, highlighting the integration of plaque and functional assessment to guide personalized treatment decisions.
Figure 2. AI-enhanced diagnostic and therapeutic pathway in stable coronary artery disease. The figure illustrates a stepwise workflow from the patient presentation with chest pain to the final treatment decision. Coronary computed tomography angiography (CCTA) is performed and processed through artificial intelligence (AI)-based techniques including AI-QCT for plaque characterization and FFR-CT for functional assessment. These outputs guide clinical decision making, resulting in either optimized medical therapy or percutaneous coronary intervention (PCI).
AI IN CORONARY ANGIOGRAPHY FOR INTERVENTIONAL CARDIOLOGY
Artificial intelligence potentially offers significant advantages to coronary angiography, with respect to lesion detection, procedural efficiency, and therapeutic decision making. Traditional angiographic assessments rely mostly on operator experience and are subject to interobserver variability and subjective interpretation. AI-based quantitative coronary angiography addresses these limitations by providing automated, real-time, and highly reproducible assessments of coronary lesions, improving diagnostic accuracy and guiding interventional strategies with greater accuracy[30].
AI-driven automated coronary lesion analysis
Automated quantitative coronary angiography (AI-QCA) employs DL algorithms trained on large angiographic datasets to automate lesion assessment, vessel diameter quantification, and real-time severity classification[31]. This technology enhances diagnostic precision by detecting subtle morphological features of atherosclerotic plaques that may be overlooked by operators. Unlike traditional edge-detection algorithms, DL-based AI continuously refines its analysis, improving lesion characterization and reducing observer variability.
Recent advancements have demonstrated that AI-QCA significantly improves coronary lesion assessment, particularly in coronary tree segmentation, stenosis measurement, and pathology detection (e.g., thrombi, dissections). A notable example is DeepDiscern, which achieved an 87.6% accuracy in recognizing coronary pathologies[32]. Moreover, AI-QCA has been validated in prospective studies, such as the FLASH study[30], which demonstrated its noninferiority to OCT-guided PCI in post-procedure stent optimization. Among 395 randomized patients, AI-QCA-assisted PCI achieved a post-PCI minimum stent area (MSA) of
Despite its potential, AI-QCA still presents limitations compared to intravascular imaging techniques such as OCT and IVUS. In the FLASH study, stent malapposition was observed more frequently in the AI-QCA group (13.6% vs. 5.6%, P = 0.007), suggesting that while AI-QCA provides rapid and automated lesion evaluation, it may not yet fully replace high-resolution intravascular imaging for post-stent optimization. Additionally, validation studies comparing AI-QCA to IVUS and manual QCA have shown moderate to strong correlations in reference vessel areas and minimal lumen area but weaker agreement in lesion length and stenosis severity assessment, likely due to geographic mismatch in lesion margins. AI-QCA also tends to underestimate lesion length, particularly in cases of diffuse atherosclerosis, although refining proximal and distal lesion definitions can improve accuracy[33].
Beyond lesion quantification, AI-based models are being developed to analyze complex angiographic patterns and predict plaque morphology, integrating radiomic features to differentiate stable from vulnerable plaques[11].
Furthermore, AI-QCA is advancing toward a more integrative approach by incorporating vessel geometry parameters, such as curvature and eccentricity, which influence hemodynamic stress and plaque vulnerability. This additional layer of analysis enhances lesion characterization beyond traditional diameter stenosis assessment, potentially refining decision making for percutaneous coronary interventions (PCI)[34].
AI-powered functional assessment in angiography
Beyond lesion quantification, AI-based models are being developed to analyze complex angiographic patterns and predict plaque morphology, integrating radiomic features to differentiate stable from vulnerable plaques[35]. At the same time, AI is broadening the diagnostic role of invasive coronary angiography beyond coronary lesion assessment. The AI-ENCODE study demonstrated that AI can extract functional and hemodynamic parameters directly from routine angiograms, including left ventricular ejection fraction (LVEF), diastolic dysfunction (LVDD), right ventricular dysfunction, and cardiac index (CI), with high predictive accuracy (AUC: 0.87, 0.87, 0.78, and 0.74, respectively)[36].
Additionally, AI-based fractional AI-FFR could support physiological lesion assessment by integrating coronary flow dynamics and computational hemodynamics. Recent models achieve 91% sensitivity, 95% specificity, and an overall accuracy of 94%, enabling near-instantaneous FFR calculation from angiographic videos in under 40 seconds with minimal manual input, facilitating routine clinical adoption[37]. In a study by Omori et al., AI-based angioFFR software trained on > 27,000 synthetic coronary geometries demonstrated 87.7% accuracy (95%CI: 83.1%-91.5%) and 94.3% specificity in detecting hemodynamically significant stenosis compared to invasive FFR[38].
A further advancement is FFRangio, an AI-based technology that calculates FFR directly from angiograms, eliminating the need for pressure wires or pharmacological hyperemia. A two-center study involving 536 patients undergoing coronary angiography showed that 91.8% received treatment aligned with FFRangio values, with 1-year MACE rates of 2.5% (deferral) and 4.1% (PCI) - comparable to invasive FFR outcomes. This approach streamlines workflow, reduces contrast load, and enhances real-time decision making in the cath lab[39]. However, clinical evidence on QFR-based techniques such as FFRangio remains mixed. The FAVOR III China trial[40] demonstrated a significant reduction in adverse cardiovascular events using QFR guidance compared to standard angiography, whereas the FAVOR III Europe study[41] failed to show noninferiority of QFR vs. pressure wire-based FFR, raising concerns about its generalizability in Western populations. These contrasting results highlight the need for further validation in diverse clinical settings.
Real-time AI integration for procedural optimization and in predicting revascularization needs
AI-powered fluoroscopy tracking reduces radiation exposure while refining operator precision, shortening procedural times, and lowering contrast use, particularly in complex anatomies[42].
By providing real-time vessel tracking, AI-enhanced imaging facilitates more accurate catheter positioning, improving navigation through lesions and optimizing device deployment. A study by Yang et al. demonstrated that DL-based models effectively identified and segmented major coronary vessels in angiographic images, achieving an average segmentation accuracy (F1 score) of 0.917[43]. Notably, 93.7% of analyzed images surpassed an F1 score of 0.8, reflecting a high level of precision in vessel delineation, enhancing QCA diagnostics and streamlining procedural workflows while maintaining accuracy[43,44].
Beyond imaging enhancements, AI-driven procedural guidance is reshaping coronary stenting by refining lesion assessment, vessel sizing, and real-time stent placement strategies. Dynamic Coronary Roadmap (DCR) technology, although not fully AI-driven, enhances coronary visualization through automated real-time vessel tracking, further optimizing procedural workflow while minimizing contrast and radiation exposure[45,46].
Early studies highlighted the potential of AI-OCT in PCI, demonstrating improved procedural decision making and a more precise assessment of stent expansion. Findings suggested AI-driven guidance could optimize stent deployment by identifying underexpansion risks and procedural factors[46,47]. More recently, Sibbald et al. reinforced these results, reporting that the Ultreon AI-OCT system led to a 2.83-fold improvement in stent sizing accuracy (P < 0.001) and significantly reduced OCT interpretation time, further validating AI’s role in standardizing PCI workflows and improving efficiency[48].
Advancements in AI-driven procedural guidance now integrate augmented reality (AR), real-time catheter tracking, and mixed reality (MR) holographic imaging, enhancing visualization and precision[49]. AR-based holographic projections are emerging as tools to optimize catheter manipulation, while MR imaging, as demonstrated by Tsai et al., enables real-time 3D measurements with high accuracy, refining surgical and interventional planning[50]. Additionally, AI-powered dynamic coronary roadmapping, leveraging Bayesian filtering for catheter tip tracking in fluoroscopy, improves intraoperative navigation by compensating for motion and reducing contrast use during PCI[51].
FUTURE PERSPECTIVES AND LIMITATIONS OF AI IN CLINICAL CARDIOLOGY
While artificial intelligence is steadily transforming interventional cardiology, its clinical adoption still faces significant, real-world challenges. Beyond the technical progress, translating AI innovations into routine practice requires navigating a complex landscape of ethical concerns, regulatory frameworks, and clinical integration hurdles. These elements are often underemphasized but crucial for ensuring that innovation translates into impact.
One rapidly evolving area is AI-powered ECG monitoring through wearable devices. These tools, supported by DL algorithms, enable earlier detection of ischemic events and arrhythmic complications - particularly relevant in the context of PCI. They promise faster clinical decision making and better procedural planning[52,53]. Similarly, AI-based clinical decision support systems (CDSS) are finding space in the post-PCI setting, where they can support drug titration, patient monitoring, and risk-based therapeutic decisions by integrating real-time, multimodal data[54,55].
However, trust remains a key ingredient for the clinical uptake of these systems. This is where explainable AI (XAI) comes into play. Techniques such as SHAP and LIME are increasingly used to make model predictions interpretable and transparent to clinicians - an essential step for real-life adoption, especially in high-stakes domains like coronary artery disease management[56,57].
The use of AI in robotic-assisted PCI (R-PCI) is another promising, yet complex frontier. While robotic platforms such as CorPath GRX and R-One have already demonstrated improvements in procedural precision and radiation safety, challenges persist. High costs, prolonged procedure times, and the need for specialized training currently limit broader uptake[58-63]. Encouragingly, the integration of AI into robotic platforms - through decision algorithms, adaptive learning, and automated wiring techniques - could support the shift toward more consistent and reproducible interventions, even in complex lesion subsets. Further forward, augmented and virtual reality platforms are being explored for training and procedural simulation, while AI-enhanced AI-QCA is showing potential in automated lesion assessment. However, both these areas require stronger validation before achieving widespread clinical credibility[64,65].
A structured overview of current applications, benefits, and limitations is presented in Table 1. Still, perhaps the most critical barrier to meaningful AI integration is algorithmic bias. AI models are often trained on datasets that do not adequately reflect real-world diversity. This poses risks - particularly for women, minorities, and socioeconomically disadvantaged patients - whose disease presentations or access to care may differ from the populations used to build predictive models[66-71].
Summary of AI Applications in Interventional Cardiology: Functions, Advantages, and Limitations
| AI application | Primary function | Key advantages | Limitations | References |
| AI-QCT | Plaque characterization, risk stratification | Reduces interobserver variability, improves plaque quantification | High computational demand, requires standardization | [7,12,13,17] |
| FFR-CT | Functional assessment of stenosis | Improves ischemia detection, refines PCI decision making | Moderate specificity in high calcified burden cases | [15,18,19,21] |
| AI-QCA | Automated lesion quantification and assessment | Enhances reproducibility, reduces procedural variability | Lesion length estimation challenges, stent malapposition | [30,32,33,35] |
| AI-FFR | Noninvasive physiological lesion assessment | Fast, real-time FFR estimation without invasive measures | Potential variability in real-world datasets | [39-42] |
| AI-OCT | Stent optimization, procedural guidance | Increases accuracy of stent placement, reduces complications | Dependent on imaging quality, limited adoption | [46-48,50] |
| AI in robotics (R-PCI) | Precision in catheter-based interventions, tele-stenting | Reduces radiation exposure, improves procedural ergonomics | High cost, longer procedure times, limited accessibility | [58,60-62] |
| AI for risk stratification | Predictive analytics for MACE and long-term prognosis | Personalized risk assessment, integrates multimodal data | Algorithmic bias, need for large validated datasets | [26-29] |
As a result, AI tools may inadvertently perpetuate or even amplify disparities in cardiovascular care. Bias can be introduced at any phase: data collection, algorithm design, testing, and deployment. The consequences are not merely academic - misclassifications, inaccurate risk estimations, or flawed treatment suggestions can have real and potentially harmful clinical outcomes[57,71]. Improving dataset quality, ensuring transparency in model training, and embedding fairness checks should become standard practice.
Figure 3 illustrates the clinical shift from a traditional, experience-based approach to an AI-assisted model incorporating imaging analysis, scoring, and PCI optimization.
Figure 3. Traditional vs. AI-Assisted Interventional Cardiology. Comparison between a conventional approach - based on physical examination, paper-based tools, and manual decision making - and a modern AI-supported workflow integrating imaging analysis, automated risk scoring, and robotic-assisted PCI (Percutaneous Coronary Intervention).
CONCLUSION
Artificial intelligence is rapidly evolving from an adjunctive tool to a central component in the management of coronary artery disease. By integrating imaging, physiological, and clinical data, AI enhances diagnostic precision, procedural planning, and personalized risk stratification. Technologies such as AI-QCA, FFR-CT, and AI-guided robotics are reshaping interventional strategies, while novel biomarkers and ML models refine outcome prediction. Despite these advances, challenges remain: data standardization, algorithm transparency, clinical integration, and ethical oversight are crucial for responsible implementation. Going forward, AI’s true impact will depend not only on innovation, but on validation, equity, and seamless adoption into routine cardiovascular care.
DECLARATIONS
Acknowledgments
The authors would like to acknowledge all those who have contributed, directly or indirectly, to the development of this work.
Authors’ contributions
Made substantial contributions to the conception and design of the study and performed data analysis and interpretation: Veneziano FA, Cocco N
Performed data acquisition, as well as providing administrative, technical, and material support:
All authors contributed to drafting and revising the manuscript, approved the final version, and agreed to be accountable for all aspects of the work.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
2. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213-24.
3. Zimmermann FM, Ferrara A, Johnson NP, et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J. 2015;36:3182-8.
4. Martin WG, McNaughton E, Bambrough PB, West NEJ, Hoole SP. Interobserver variability between expert, experienced, and novice operator affects interpretation of optical coherence tomography and 20 MHz intravascular ultrasound imaging. Cardiovasc Revasc Med. 2023;47:33-9.
5. Parsa S, Somani S, Dudum R, Jain SS, Rodriguez F. Artificial intelligence in cardiovascular disease prevention: is it ready for prime time? Curr Atheroscler Rep. 2024;26:263-72.
6. Vidal-Perez R, Vazquez-Rodriguez JM. Role of artificial intelligence in cardiology. World J Cardiol. 2023;15:116-8.
7. Omori H, Matsuo H, Fujimoto S, et al. Determination of lipid-rich plaques by artificial intelligence-enabled quantitative computed tomography using near-infrared spectroscopy as reference. Atherosclerosis. 2023;386:117363.
8. El Sherbini A, Rosenson RS, Al Rifai M, et al. Artificial intelligence in preventive cardiology. Prog Cardiovasc Dis. 2024;84:76-89.
9. Choi AD, Marques H, Kumar V, et al. CT evaluation by artificial intelligence for atherosclerosis, stenosis and vascular morphology (CLARIFY): a multi-center, international study. J Cardiovasc Comput Tomogr. 2021;15:470-6.
10. Zhang Y, Feng Y, Sun J, et al. Fully automated artificial intelligence-based coronary CT angiography image processing: efficiency, diagnostic capability, and risk stratification. Eur Radiol. 2024;34:4909-19.
11. Moon IT, Kim SH, Chin JY, et al. Accuracy of artificial intelligence-based automated quantitative coronary angiography compared to intravascular ultrasound: retrospective cohort study. JMIR Cardio. 2023;7:e45299.
12. Rinehart S, Raible SJ, Ng N, et al. Utility of artificial intelligence plaque quantification: results of the DECODE study. J Soc Cardiovasc Angiogr Interv. 2024;3:101296.
13. van Assen M, von Knebel Doeberitz P, Quyyumi AA, De Cecco CN. Artificial intelligence for advanced analysis of coronary plaque. Eur Heart J Suppl. 2023;25:C112-7.
14. Lin A, Kolossváry M, Cadet S, et al. Radiomics-based precision phenotyping identifies unstable coronary plaques from computed tomography angiography. JACC Cardiovasc Imaging. 2022;15:859-71.
15. Lipkin I, Telluri A, Kim Y, et al. Coronary CTA with AI-QCT interpretation: comparison with myocardial perfusion imaging for detection of obstructive stenosis using invasive angiography as reference standard. AJR Am J Roentgenol. 2022;219:407-19.
16. Kim Y, Choi AD, Telluri A, et al. Atherosclerosis imaging quantitative computed tomography (AI-QCT) to guide referral to invasive coronary angiography in the randomized controlled CONSERVE trial. Clin Cardiol. 2023;46:477-83.
17. Khan H, Bansal K, Griffin WF, et al. Assessment of atherosclerotic plaque burden: comparison of AI-QCT versus SIS, CAC, visual and CAD-RADS stenosis categories. Int J Cardiovasc Imaging. 2024;40:1201-9.
18. Peters B, Paul JF, Symons R, Franssen WMA, Nchimi A, Ghekiere O. Invasive fractional-flow-reserve prediction by coronary CT angiography using artificial intelligence vs. computational fluid dynamics software in intermediate-grade stenosis. Int J Cardiovasc Imaging. 2024;40:1875-80.
19. Narimani-Javid R, Moradi M, Mahalleh M, et al. Machine learning and computational fluid dynamics derived FFRCT demonstrate comparable diagnostic performance in patients with coronary artery disease: a systematic review and meta-analysis. Eur Heart J. 2025;19:232-46.
20. Li Y, Yu M, Dai X, et al. Detection of hemodynamically significant coronary stenosis: CT myocardial perfusion versus machine learning CT fractional flow reserve. Radiology. 2019;293:305-14.
21. Chiou A, Hermel M, Sidhu R, et al. Artificial intelligence coronary computed tomography, coronary computed tomography angiography using fractional flow reserve, and physician visual interpretation in the per-vessel prediction of abnormal invasive adenosine fractional flow reserve. Eur Heart J Imaging Methods Pract. 2024;2:qyae035.
22. Dundas J, Leipsic J, Fairbairn T, et al. Interaction of AI-enabled quantitative coronary plaque volumes on coronary CT angiography, FFR(CT), and clinical outcomes: a retrospective analysis of the ADVANCE registry. Circ Cardiovasc Imaging. 2024;17:e016143.
23. Li J, Yang Z, Sun Z, et al. CT coronary fractional flow reserve based on artificial intelligence using different software: a repeatability study. BMC Med Imaging. 2024;24:288.
24. Guo B, Jiang M, Guo X, et al. Diagnostic and prognostic performance of artificial intelligence-based fully-automated on-site CT-FFR in patients with CAD. Sci Bull. 2024;69:1472-85.
25. Yang J, Shan D, Wang X, et al. On-site computed tomography-derived fractional flow reserve to guide management of patients with stable coronary artery disease: the TARGET randomized trial. Circulation. 2023;147:1369-81.
26. Jaltotage B, Lu J, Dwivedi G. Use of artificial intelligence including multimodal systems to improve the management of cardiovascular disease. Can J Cardiol. 2024;40:1804-12.
27. Chan K, Wahome E, Tsiachristas A, et al. Inflammatory risk and cardiovascular events in patients without obstructive coronary artery disease: the ORFAN multicentre, longitudinal cohort study. Lancet. 2024;403:2606-18.
28. Napoli G, Pergola V, Basile P, et al. Epicardial and pericoronary adipose tissue, coronary inflammation, and acute coronary syndromes. J Clin Med. 2023;12:7212.
29. Aromiwura AA, Settle T, Umer M, et al. Artificial intelligence in cardiac computed tomography. Prog Cardiovasc Dis. 2023;81:54-77.
30. Kim Y, Yoon HJ, Suh J, et al. Artificial intelligence-based fully automated quantitative coronary angiography vs optical coherence tomography-guided PCI: the FLASH trial. JACC Cardiovasc Interv. 2025;18:187-97.
31. Chae J, Kweon J, Park GM, et al. Enhancing quantitative coronary angiography (QCA) with advanced artificial intelligence: comparison with manual QCA and visual estimation. Int J Cardiovasc Imaging. 2025;41:559-68.
32. Howard J, Reiber JHC. Automated analysis of coronary angiograms using artificial intelligence: a window into the cath lab of the future. EuroIntervention. 2021;17:16-7.
33. Gautam N, Saluja P, Malkawi A, et al. Current and future applications of artificial intelligence in coronary artery disease. Healthcare. 2022;10:232.
34. Nobre Menezes M, Oliveira CS, Silva JL, et al. Old habits die hard: can AI help bring coronary angiography into the 21st century? JACC Adv. 2024;3:101093.
35. Hae H, Kang SJ, Kim WJ, et al. Machine learning assessment of myocardial ischemia using angiography: Development and retrospective validation. PLoS Med. 2018;15:e1002693.
36. Alkhouli M, Rostami B, Attia Z, Friedman PA, Gulati R. LB-3|Artificial intelligence for extracting non-COronary data from angiography: the AI-ENCODE study. J Soc Cardiovasc Angiogr Interv. 2024;3:101870.
37. Ben-Assa E, Abu Salman A, Cafri C, et al. Performance of a novel artificial intelligence software developed to derive coronary fractional flow reserve values from diagnostic angiograms. Coron Artery Dis. 2023;34:533-41.
38. Omori H, Kawase Y, Mizukami T, et al. Diagnostic accuracy of artificial intelligence-based angiography-derived fractional flow reserve using pressure wire-based fractional flow reserve as a reference. Circ J. 2023;87:783-90.
39. Witberg G, Bental T, Levi A, et al. Clinical outcomes of FFRangio-guided treatment for coronary artery disease. JACC Cardiovasc Interv. 2022;15:468-70.
40. Xu B, Tu S, Song L, et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): a multicentre, randomised, sham-controlled trial. Lancet. 2021;398:2149-59.
41. Andersen BK, Sejr-Hansen M, Maillard L, et al. Quantitative flow ratio versus fractional flow reserve for coronary revascularisation guidance (FAVOR III Europe): a multicentre, randomised, non-inferiority trial. Lancet. 2024;404:1835-46.
42. Ploscaru V, Popa-Fotea NM, Calmac L, et al. Artificial intelligence and cloud based platform for fully automated PCI guidance from coronary angiography-study protocol. PLoS One. 2022;17:e0274296.
43. Yang S, Kweon J, Roh JH, et al. Deep learning segmentation of major vessels in X-ray coronary angiography. Sci Rep. 2019;9:16897.
44. Aminorroaya A, Biswas D, Pedroso AF, Khera R. Harnessing artificial intelligence for innovation in interventional cardiovascular care. J Soc Cardiovasc Angiogr Interv. 2025;4:102562.
45. Behnoush AH, Ramandi A, Mahajan S, Altibi A, Samavarchitehrani A, Gupta R. Dynamic coronary roadmap in percutaneous coronary intervention: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2024;24:681.
46. Bartuś S, Siłka W, Kasprzycki K, et al. Experience with optical coherence tomography enhanced by a novel software (Ultreon™ 1.0 software)-the first one hundred cases. Medicina. 2022;58:1227.
47. Januszek R, Siłka W, Sabatowski K, et al. Procedure-related differences and clinical outcomes in patients treated with percutaneous coronary intervention assisted by optical coherence tomography between new and earlier generation software (Ultreon™ 1.0 software vs. AptiVue™ software). J Cardiovasc Dev Dis. 2022;9:218.
48. Sibbald M, Mitchell HR, Buccola J, Pinilla-Echeverri N. Impact of artificial intelligence-enhanced optical coherence tomography software on percutaneous coronary intervention decisions. J Soc Cardiovasc Angiogr Interv. 2025;4:102438.
49. Xu B, Zhang R. Virtual PCI powered by augmented reality: pave the way to optimal revascularization. JACC Cardiovasc Interv. 2023;16:795-7.
50. Tsai TY, Kageyama S, He X, et al. Feasibility and accuracy of real-time 3D-holographic graft length measurements. Eur Heart J Digit Health. 2024;5:101-4.
51. Ma H, Smal I, Daemen J, Walsum TV. Dynamic coronary roadmapping via catheter tip tracking in X-ray fluoroscopy with deep learning based Bayesian filtering. Med Image Anal. 2020;61:101634.
52. Gala D, Behl H, Shah M, Makaryus AN. The role of artificial intelligence in improving patient outcomes and future of healthcare delivery in cardiology: a narrative review of the literature. Healthcare. 2024;12:481.
53. Singh M, Kumar A, Khanna NN, et al. Artificial intelligence for cardiovascular disease risk assessment in personalised framework: a scoping review. EClinicalMedicine. 2024;73:102660.
54. Neri L, Oberdier MT, van Abeelen KCJ, et al. Electrocardiogram monitoring wearable devices and artificial-intelligence-enabled diagnostic capabilities: a review. Sensors. 2023;23:4805.
55. Lee S, Chu Y, Ryu J, Park YJ, Yang S, Koh SB. Artificial intelligence for detection of cardiovascular-related diseases from wearable devices: a systematic review and meta-analysis. Yonsei Med J. 2022;63:S93-S107.
56. Bozyel S, Şimşek E, Koçyiğit Burunkaya D, et al. Artificial intelligence-based clinical decision support systems in cardiovascular diseases. Anatol J Cardiol. 2024;28:74-86.
57. Olawade DB, Aderinto N, Olatunji G, Kokori E, David-Olawade AC, Hadi M. Advancements and applications of Artificial Intelligence in cardiology: current trends and future prospects. J Med Surg Public Health. 2024;3:100109.
58. Sadeghi Z, Alizadehsani R, Cifci MA, et al. A review of explainable artificial intelligence in healthcare. Comput Electr Eng. 2024;118:109370.
59. Alkhanbouli R, Matar Abdulla Almadhaani H, Alhosani F, Simsekler MCE. The role of explainable artificial intelligence in disease prediction: a systematic literature review and future research directions. BMC Med Inform Decis Mak. 2025;25:110.
60. Rudnicka Z, Pręgowska A, Glądys K, Perkins M, Proniewska K. Advancements in artificial intelligence-driven techniques for interventional cardiology. Cardiol J. 2024;31:321-41.
61. Maor E, Eleid MF, Gulati R, Lerman A, Sandhu GS. Current and future use of robotic devices to perform percutaneous coronary interventions: a review. J Am Heart Assoc. 2017;6:e006239.
62. Khokhar AA, Marrone A, Bermpeis K, et al. Latest developments in robotic percutaneous coronary interventions. Interv Cardiol. 2023;18:e30.
63. Hofmann FJ, Dörr O, Blachutzik F, et al. Latest developments in robotic percutaneous coronary intervention. Surg Technol Int. 2021;38:325-30.
64. Durand E, Eltchaninoff H. Robotic-assisted percutaneous coronary intervention: the future or the past? EuroIntervention. 2024;20:19-20.
65. Rajakumar HK. Is artificial intelligence-based quantitative coronary angiography ready for clinical adoption? Ind J Clin Cardiol. 2025;6:92-3.
66. Samant S, Bakhos JJ, Wu W, et al. Artificial intelligence, computational simulations, and extended reality in cardiovascular interventions. JACC Cardiovasc Interv. 2023;16:2479-97.
67. Mihan A, Pandey A, Van Spall HGC. Artificial intelligence bias in the prediction and detection of cardiovascular disease. NPJ Cardiovasc Health. 2024;1:31.
68. Mihan A, Pandey A, Van Spall HG. Mitigating the risk of artificial intelligence bias in cardiovascular care. Lancet Digit Health. 2024;6:e749-54.
69. Assen M, Beecy A, Gershon G, Newsome J, Trivedi H, Gichoya J. Implications of bias in artificial intelligence: considerations for cardiovascular imaging. Curr Atheroscler Rep. 2024;26:91-102.
70. Nolin-Lapalme A, Corbin D, Tastet O, Avram R, Hussin JG. Advancing fairness in cardiac care: strategies for mitigating bias in artificial intelligence models within cardiology. Can J Cardiol. 2024;40:1907-21.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].